Abstract
To evaluate the repeatability of a novel automated technique called Smart ERA (Smart Endometrial Receptivity Analysis) for the automated segmentation and volume calculation of the endometrium in patients with normal uteri,, and to compare the agreement of endometrial volume measurements between Smart ERA, the semi-automated Virtual Organ Computer-aided Analysis (VOCAL) technique and manual segmentation. This retrospective study evaluated endometrial volume measurement in infertile patients who underwent frozen-thawed embryo transfer (FET). Transvaginal three-dimensional ultrasound scans were performed using a Resona R9 ultrasound machine. Data was collected from patients between 2021 and 2022. Patients with normal uteri and optimal ultrasound images were included. Endometrial volumes were measured using Smart ERA, VOCAL at 15° rotation, and manual segmentation. Intra-observer repeatability and agreement between techniques were assessed using the intraclass correlation coefficient (ICC) and Bland–Altman analysis. A total of 407 female patients were evaluated (mean age 33.2 ± 4.7 years). The repeatability of Smart ERA showed an ICC of 0.983 (95% CI 0.984–0.991). The agreement between Smart ERA and the manual method, Smart ERA and VOCAL, and VOCAL and the manual method, as assessed by ICC, were 0.986 (95% CI 0.977–0.990), 0.943 (95% CI 0.934–0.963), and 0.951 (95% CI 0.918–0.969), respectively. The Smart ERA technique required approximately 3 s for endometrial volume calculation, while VOCAL took around 5 min and the manual segmentation method took approximately 50 min. The Smart-ERA software, which employs a novel three-dimensional segmentation algorithm, demonstrated excellent intra-observer repeatability and high agreement with both VOCAL and manual segmentation for endometrial volume measurement in women with normal uteri. However, these findings should be interpreted with caution, as the algorithm's performance may not be generalizable to populations with different uterine characteristic. Additionally, Smart ERA required significantly less time compared to VOCAL and manual segmentation.
Similar content being viewed by others
Introduction
Personalizing embryo transfer timing based on endometrial receptivity represents an opportunity to significantly improve implantation rates and pregnancy outcomes in assisted reproduction1,2.
Ultrasound imaging, due to its non-invasive nature, convenience, and ability to perform synchronized assessments at different stages of the reproductive cycle, is extensively used in assisted reproduction3. While endometrial thickness is routinely assessed, it only provides a crude measure of endometrial health and fails to fully capture three-dimensional endometrial morphology. Emerging evidence suggests that endometrial volume assessed via three-dimensional ultrasound may serve as a more informative marker of receptivity.
Several studies have found lower pre-transfer endometrial volumes correlate with lower pregnancy rates4,5,6. For instance, Lam4 reported that some studies suggested the endometrium must attain a volume of at least 2.0–2.5 ml to achieve a pregnancy, Raga5 reported no pregnancies for volumes under 2 ml, while Yaman6 reported no pregnancies for volumes under 2.5 ml. Accurately measuring endometrial volume thus holds relevance for optimizing the timing of embryo transfer. Three-dimensional ultrasound with volume reconstruction allows precise quantification of the irregularly shaped endometrial cavity7,8,9. Reconstruction techniques like Virtual Organ Computer-aided Analysis (VOCAL) currently dominate clinical practice but rely on manual contouring, introducing inter-observer variability10,11,12,13,14.
An automated volume calculation tool could address this limitation and enable more consistent, operator-independent assessment. Smart-ERA is a novel software developed by Mindray Bio-Medical Electronics Co. that applies automated segmentation to identify the endometrial border and compute volume from three-dimensional ultrasound scans. However, its accuracy and reliability compared to manual delineation have not yet been systematically evaluated.
This study aimed to validate Smart ERA software for endometrial volume measurement by comparing its outputs to the manual VOCAL method. The specific objectives were to assess the time required for endometrial volume calculation using Smart ERA and manual VOCAL. Evaluate the agreement between Smart ERA and manual VOCAL for endometrial volume measurements. Determine the accuracy of Smart ERA using manual contouring on individual ultrasound slices as a reference standard. Demonstrating non-inferior performance of Smart ERA automated approach could support its integration into clinical practice to standardize endometrial assessment and optimize embryo transfer timing.
Methods
Ethical considerations
This study received approval from the Ethics Committee of Peking University Shenzhen Hospital (Approval Number: 2023028). Given the retrospective design of the study, the requirement for individual patient consent was waived. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study design and participants
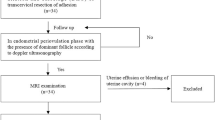
This retrospective observational study evaluated infertile patients who underwent frozen-thawed embryo transfer (IVF-ET) at the Reproductive Medicine Center of Peking University Shenzhen Hospital between 2021 and 2022. Patients received embryo transfers in either natural or hormone-downregulated cycles.
Exclusion criteria included patients with uterine abnormalities such as endometrial polyps, submucous fibroids, intrauterine adhesions, or fluid in the uterine cavity. Additionally, patients with uterine malformations, adenomyosis, or intramural fibroids that could alter the uterine cavity shape were excluded. Patients who could not obtain optimal three-dimensional ultrasound images or had indistinct endometrial and myometrial borders during three-dimensional ultrasonography were also excluded.
Ultrasound examination and data collection
The Neuwa R9 color Doppler ultrasound machines (Mindray Bio-Medical Electronics Co. Ltd.) with a transvaginal probe (DE10-3WU) operating at 2.0–9.0 MHz was employed for this study. All patients underwent two-dimensional and three-dimensional imaging. Device settings were customized for each patient. Physicians with over 5 years of experience in transvaginal gynecological ultrasound examinations performed all ultrasound examinations and 3D data acquisitions.
Examinations took place 1–4 h before embryo transfer after patients voided and assumed the lithotomy position. Endometrial thickness was measured in the sagittal view, displaying the entire cervix length and maximum uterine cavity length. The maximum thickness included both endometrial layers but excluded surrounding hyperechoic areas, measured at the endometrial-myometrial junction15.
For 3D data acquisition, the Smart Scene 3D function automatically adjusted the sampling frame to fully cover the uterine contour, following with an automatic 3D scan. This process was repeated after a 5–10 min interval while keeping the probe fixed. Acquired volume data was stored for processing and analysis.
Endometrial volume ultrasonography measurement
The Smart-ERA, VOCAL, and manual region of interest delineation and segmentation slice-by-slice (Hereinafter referred to as manual region) methods were utilized to calculate endometrial volume in each 3D dataset.
Smart ERA
The Smart ERA technology, which is integrated into the ultrasound scanner (Mindray Bio-Medical Electronics Co., Ltd.), employs a three-dimensional segmentation algorithmic framework, as depicted in Fig. 1. Initially, the three-dimensional volumetric data is resampled into multiple two-dimensional sections radially. Subsequently, a clinical prior knowledge (which refers to the experience and expertise of clinical professionals in endometrial assessment) based image segmentation algorithm is applied to segment the above two-dimensional sections and generate a two-dimensional segmentation mask of the uterine endometrium. Finally, a three-dimensional contour fitting is performed on the two-dimensional mask to obtain the three-dimensional segmentation mask, representing the uterine endometrial three-dimensional segmentation result.
Three-dimensional segmentation algorithmic framework employed by Smart ERA. Smart ERA technology utilizes an advanced three-dimensional segmentation algorithmic framework. Initially, the volumetric data is transformed into multiple radial two-dimensional sections. Following this, a sophisticated image segmentation algorithm, which incorporates comprehensive clinical prior knowledge, is applied to these two-dimensional sections. This process generates a precise two-dimensional segmentation mask of the uterine endometrium. Finally, a complex three-dimensional contour fitting is performed on the two-dimensional mask, resulting in the creation of a highly accurate three-dimensional segmentation mask.
Upon obtaining the uterine endometrial three-dimensional contour, the direction of the coronal plane of the uterine endometrium is calculated based on the three-dimensional contour. The coronal plane reference line is then automatically established using the Curved Multiplanar Reformation (CMPR) technique, which is a 3D medical image post-processing technique that allows users to define a curved path through a 3D image dataset and generate a series of parallel 2D cross-sectional images along this path to display the transverse views of the anatomical structure or lesion of interest. Furthermore, parameters such as volume, thickness, and blood flow perfusion index of the uterine endometrium can be calculated based on the uterine endometrial three-dimensional contour. The entire Smart ERA process can be completed within 2–3 s.
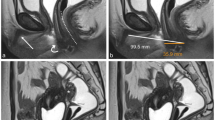
Upon visualizing the maximum sagittal plane of the uterus, the clinician initiated the Smart ERA function, prompting the ultrasound system to automatically calibrate the dimensions of the volumetric sampling box to encompass the entirety of the uterine contour. Automated completion of a three-dimensional scan and calculation of the endometrial volume is then achieved, displaying the uterine longitudinal axis (A plane), uterine coronal plane (B plane), and endometrial volume (C plane) in three orthogonal planes (Fig. 2).
Automated calculation of endometrial volume by Smart ERA. The Smart ERA system swiftly completes a three-dimensional scan automatically, efficiently recognizes, and calculates the endometrial volume. This process results in the simultaneous display of the uterine longitudinal axis (A plane), uterine coronal plane (B plane), and endometrial volume (C plane) in three orthogonal planes.
VOCAL
The VOCAL technique employs a volumetric methodology wherein two-dimensional contours are either manually delineated or automatically generated on multiple rotated planes around a fixed axis to produce a three-dimensional volume measurement. The software offers rotational angles of 6°, 9°, 15°, and 30° per contour plane, indirectly influencing the number of rotation steps required for the measurement. In the present evaluation, three intersecting planes were concurrently displayed and all measurements were performed in the mid-sagittal uterine plane. Boundary markers were positioned at the uterine cavity edges and a rotational angle of 15° was selected. The observer manually outlined the endometrium on each rotated plane until completing a 180° rotation around the vertical axis. Subsequently, the software automatically computed the endometrial volume (Fig. 3).
Semi-automated measurement of endometrial volume using the VOCAL technique in the mid-sagittal uterine plane. The VOCAL technique simultaneously presents three intersecting planes. All measurements are conducted in the mid-sagittal uterine plane. Boundary markers are strategically placed at the edges of the uterine cavity, and a rotational angle of 15° is chosen. The observer manually traces the endometrium on each rotated plane, completing a full 180° rotation around the vertical axis. Subsequently, the software automatically calculates the endometrial volume.
Manual region of interest delineation and segmentation slice by slice
The patient's three-dimensional endometrial data were exported in Digital Imaging and Communications in Medicine format via an established picture archiving and communication system. The data were then imported into the Insight Toolkit SNAP software package (Version 3.8.0-beta) where two observers with over three years experiences manually delineated the endometrium on a layer-by-layer basis to obtain a representative three-dimensional region of interest of the endometrial volume. In cases of disparate contours, a final contour resulted from consultation between the observers. The segmentation procedure is illustrated in Fig. 4. The region of interest data derived from contouring were used to compute endometrial volume, equaling the product of the contour-defined area and the physical volume corresponding to each pixel. The physical volume originated from the original ultrasound acquisition data.
Manual delineation and segmentation of the endometrium on a slice-by-slice basis. The data were imported into the Insight Toolkit SNAP software package (Version 3.8.0-beta). Here, the endometrium was manually delineated and segmented slice by slice to obtain a representative three-dimensional region of interest for the endometrial volume. The region of interest data, derived from meticulous contouring, were used to compute the endometrial volume. This volume is equal to the product of the contour-defined area and the physical volume corresponding to each pixel, which originated from the original ultrasound acquisition data.
Statistics analysis
The Kolmogorov–Smirnov test was utilized to ascertain if the metric data adhered to a normal distribution. Data that followed a normal distribution are presented as the mean and standard deviation (SD), whereas data that did not follow a normal distribution are expressed as the median and interquartile range (IQR).
Repeatability in this study refers to the consistency of measurements obtained by the same observer on the same subject at different time points. The intraclass correlation coefficient (ICC) was used to measure intra-observer repeatability. The intra-observer variability of the Smart ERA technique was assessed using Bland–Altman analysis. The agreement between different methods was assessed using ICCs. Additionally, the variability between the three endometrial volume measurement methods was evaluated using Bland–Altman analysis. An ICC above 0.7 is generally considered to indicate sufficient reliability16.
The time required for each technique was measured using a standardized stopwatch method. For each measurement, the timing started when the observer initiated the volume calculation process and stopped when the final endometrial volume was displayed. This process was repeated for each method (Smart ERA, VOCAL, and manual segmentation) across all patient datasets. The average time for each method was then calculated. The collected times were compared using either the Student’s t-test or the Wilcoxon signed-rank test, depending on the distribution of the data. A P-value less than 0.01 was considered statistically significant. All analyses were performed using SPSS version 26.0.
Results
Study population
In this study, three-dimensional ultrasound image data were collected from a total of 450 patients undergoing in vitro fertilization-embryo transfer (IVF-ET) cycles. After excluding 43 patients due to suboptimal image quality (e.g., mid-position uterus, post-cesarean section uterus), the final analysis included data from 407 patients (90.4% of the total sample). The mean age of the 407 female patients was 33.2 years with a standard deviation (SD) of 4.7 years, ranging from 21 to 47 years. Endometrial characteristics of the study population are summarized in Table 1.
Endometrial volume
Endometrial volume measurements ranged from 1.0 to 10.7 cm3. The Smart ERA and VOCAL techniques yielded larger measurements compared to the manual region technique, with mean values (± SD) of 4.45 ± 1.74 cm3, 4.56 ± 1.78 cm3, and 4.35 ± 1.71 cm3, respectively. Figure 5 illustrates endometrial volume measurements obtained using the three different techniques.
Repeatability of smart ERA
The repeatability analysis included 209 patients. No statistically significant difference was found in endometrial volume measurements obtained by the Smart ERA observer on two separate occasions (P = 0.143, Wilcoxon signed-rank test). The Smart ERA technology demonstrated high repeatability with an intraclass correlation coefficient (ICC) of 0.988 (95% confidence interval 0.984–0.991) (Table 2). The Bland–Altman plot (Fig. 6A) showed that most data points fell within the 95% CI (P < 0.005), with a slight bias towards higher values on the second measurement. The mean difference was 0.04 cm3 (95% CI − 0.61 to 0.70 cm3), indicating negligible bias.
Bland–Altman plots comparing endometrial volume measurements between different methods. (A) The Bland–Altman plot demonstrates a high level of consistency, as the majority of data points fall within the 95% confidence interval. Notably, there is a higher concentration of data points above the average difference line compared to those below it. (B) The plot reveals a mean difference of 0.10 cm3 between the Smart ERA and the manual method, indicating a close agreement between these two techniques. (C) Similarly, the mean difference between the Smart ERA and VOCAL (15°) is slightly higher at 0.11 cm3, yet still suggests a strong correlation. (D) The plot also shows that the mean difference in volumetric measurements across all techniques is a mere 0.22 cm3, further supporting the impressive consistency of these methods.
Agreement among techniques
The agreement analysis included 198 patients. High ICCs were observed between any two of the Smart ERA, VOCAL (15°) and manual region techniques, with the highest ICC between Smart ERA and the manual technique (Table 2). The manual technique consistently yielded the smallest volume measurements (P < 0.001, Wilcoxon signed-rank test). However, agreement between any two of the three techniques was high.
Smart ERA versus manual region of interest delineation and segmentation slice-by-slice
A statistically significant difference was found between Smart ERA and manual region technique measurements (P < 0.001, Wilcoxon test). However, there was good agreement (ICC = 0.986, 95% CI 0.977–0.990, P < 0.001). The Bland–Altman plot (Fig. 6B) showed a mean difference of 0.10 cm3 (95% CI − 0.64 to 0.43 cm3).
Smart ERA versus VOCAL
Volumetric measurements differed significantly between Smart ERA and VOCAL (15°) (P < 0.001, Wilcoxon test), but showed good agreement (ICC = 0.951, 95% CI 0.934–0.963, P < 0.001). The Bland–Altman plot (Fig. 6C) revealed a mean difference of 0.11 cm3 (95% CI − 1.18 to 0.95 cm3).
VOCAL versus manual region of interest delineation and segmentation slice-by-slice
Significant differences were found between VOCAL (15°) and manual region technique measurements (P < 0.001, Wilcoxon test), but agreement remained good (ICC = 0.951, 95% CI 0.918–0.969, P < 0.001). The Bland–Altman plot (Fig. 6D) showed a mean difference of 0.22 cm3 (95% CI − 1.20 to 0.77 cm3).
Time required for measuring endometrial volume
As shown in Table 3, the time required for volume measurement varied significantly among the three techniques. Smart ERA and VOCAL required substantially less time than the manual region technique (P < 0.001). Smart ERA consistently took approximately 3 s per dataset, VOCAL approximately 5 min, and the manual region technique approximately 50 min. Increasing the number of slices and rotation steps correlated with longer measurement times. Using the same number of slices and rotation step length, the VOCAL technique faster than the manual region technique (P < 0.001).
Discussion
The results demonstrate high intra-observer repeatability and agreement among the three endometrial volume measurement techniques. A key finding was the exceptional concordance between mean endometrial volumes obtained using Smart ERA VOCAL and manual segmentation, indicating that Smart ERA provides an accurate assessment of endometrial size. The high ICC of 0.983 for Smart ERA suggests that repeat measurements by the same observer yielded almost identical volumetric results. Such superior intra-observer repeatability is indispensable for a volumetric analysis approach to gain acceptance in clinical practice settings where consistency is paramount. Moreover, good agreement was found when comparing Smart ERA to VOCAL and manual segmentation. This suggests automated endometrial volumetry using Smart ERA yields repeatable and consistent measurements. The minor volume differences between methods likely stem from Smart ERA's fully automated contouring versus the user-dependent nature of VOCAL and manual segmentation. Nevertheless, the small mean differences within 1 ml highlight Smart ERA's ability to approximate manual delineation. The agreement between Smart ERA and the other reference techniques surpassing 0.951 further corroborate Smart ERA as a valid alternative for obtaining endometrial volumes.
Previous studies have established VOCAL and manual outlining as standard techniques, reporting intra- and inter-observer reliability over 0.917,18,19. However, their major limitations are the protracted analysis times—around 5 min for VOCAL and over 50 min on average for manual delineation. When processing large datasets in research or managing heavy caseloads clinically, these timeframes become highly impractical. A seminal finding of the current study was that Smart ERA required only approximately 3 s on average per scan. This much faster speed compared to existing methods, achieved without compromising reliability or agreement, represents a major advancement. By drastically reducing workload, Smart ERA opens new possibilities that were previously unfeasible due to time constraints alone. For example, endometrial volume is an important parameter for assessing endometrial receptivity, and the efficiency of Smart ERA enables endometrial volumes to be rapidly processed intra-operatively as part of surgical embryo transfer procedures. This could guide treatment decisions like optimal timing based on real-time evaluation of endometrial development and receptivity on the day of transfer.
These results are concordant with existing literature that favors automated volume quantification over manual approaches for objects with irregular shapes20. Manual contouring is susceptible to subjectivity and time expenditure, while algorithms can apply consistent mathematical rules objectively. Previously, Raine-Fenning et al.18 and Yaman et al.21 found automated rotating planar contouring diminished inter-observer variation in endometrial volume computations versus manual tracing methods, though some bias remained. The current study significantly advances the field by demonstrating near-perfect intra-observer reliability with Smart ERA, likely attributable to its incorporation of specialized clinical knowledge into the segmentation algorithm. Furthermore, Smart ERA provides an automated approach, avoiding potential inconsistencies from manual tracing variability between observers or across time. This propels volumetric analysis towards improved efficiency, consistency and clinical practicality compared to semi-automated or manual modalities.
To our knowledge, Smart ERA stands out as one of the few automated methods for measuring endometrial volume. Although there are other AI-based algorithms explored in the scientific literature that focus on measuring endometrial thickness and rely on segmentation, such as the methods presented by Hu et al. (2019), and Wang et al. (2022), Smart ERA differentiates itself by providing a comprehensive volume measurement rather than just thickness. These comparisons highlight the need for further studies to directly compare the performance of Smart ERA with these AI-based segmentation algorithms. Comparison with the reference standard manual segmentation corroborated Smart ERA's ability to precisely delineate anatomical boundaries with strong agreement. While future studies in diverse populations are needed, preliminary evidence strongly suggests Smart ERA may offer clinicians a standardized and efficient means for evaluating the endometrium when time is constrained in busy clinical settings. Its rapid and consistent measurements could facilitate more routine integration of endometrial assessment into treatment planning and cycle monitoring for infertile patients.
Moreover, Smart ERA renders large multicenter studies and meta-analyses linking endometrial parameters to reproductive outcomes far more feasible. Prior investigation revealed associations between endometrial thickness/volume and implantation, but sample sizes were modest owing to manual outlining burdens. With Smart ERA, high sample trials are practical for conclusively establishing diagnostic or prognostic cutoff thresholds with adequate statistical power. Validated biomarkers could then form the foundation for personalized IVF protocols tailored according to each patient's endometrial characteristics. This paradigm shift towards individualization driven by big data holds great potential to maximize treatment success rates.
This study bears limitations common to retrospective analyses. Primarily, intra-observer variability alone was evaluated for Smart ERA while inter-observer agreement between sonographers was not. Without inter-observer reliability data, the potential for operator variability remains uncertain. Assessing multiple clinicians performing Smart ERA measurements would help establish its robustness between users. Secondly, the study population solely included patients undergoing IVF-ET without endometrial pathology. Smart ERA's accuracy in the presence of conditions such as polyps, hyperplasia or cancer, which could challenge the segmentation algorithm, requires validation. Larger cohorts representing diverse pathologies are needed. Thirdly, relying on stored ultrasound clips rather than concurrent measurements in the retrospective design may have impacted consistency between techniques. Prospective head-to-head comparisons simultaneously applying each method are warranted. Lastly, it is crucial to note that 43 out of 450 patients (9.6%) were excluded from our analysis due to suboptimal image quality. This exclusion may limit the generalizability of our findings to a broader patient population. This exclusion was primarily due to the presence of a flat uterus or a history of cesarean section, which posed challenges in obtaining clear and distinct uterine endometrium boundaries on ultrasound. In these cases, the uterus lies in a plane parallel to the ultrasound beam. This parallel orientation, coupled with the increased distance between the probe and the uterus, particularly in the case of a post-cesarean section uterus where the anterior uterine wall is often adhered to the abdominal wall, can lead to a significant degradation of image quality. The resulting images often exhibit poor tissue contrast and indistinct boundaries between the endometrium and myometrium, making accurate segmentation challenging. Under such circumstances, both the Smart-ERA and VOCAL techniques, which rely on clear delineation of the endometrial-myometrial interface, may encounter difficulties in accurately segmenting the endometrium. Further research is warranted to explore alternative imaging techniques or algorithmic adjustments that could potentially mitigate these challenges and expand the applicability of Smart-ERA to a broader spectrum of patients. Addressing these limitations via well-designed prospective trials incorporating varied populations could optimize and advance Smart ERA as an endometrial assessment tool. Additionally, the modest sample of 407 patients and two observers may limit generalizability. Inter-observer reliability was not evaluated. Broader multicenter studies involving multiple sonographers across diverse clinical settings are imperative to fully validate Smart ERA volumetry's general consistency. Standardizing image acquisition may also help optimize repeatability between centers. Continued research to refine and reinforce the algorithm's segmentation performance could bolster robustness.
Conclusion
This comprehensive study has convincingly demonstrated that Smart-ERA enables rapid and reliable endometrial volumetric quantification, exhibiting exceptional concordance with established techniques. The remarkably reduced analysis time afforded by Smart-ERA significantly enhances its potential for seamless integration into busy IVF clinical practice settings. Furthermore, Smart-ERA's high accuracy and efficiency position it as a promising tool for advancing research in reproductive medicine, potentially facilitating larger-scale studies and more personalized treatment approaches in the future.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Miravet-Valenciano, J., Ruiz-Alonso, M., Simón, C. Transcriptomics of the Human Endometrium and Embryo Implantation. Human Reproductive and Prenatal Genetics 271–291 (Academic Press, 2019).
Singh, N. et al. Can endometrial volume assessment predict the endometrial receptivity on the day of hCG trigger in patients of fresh IVF cycles: A prospective observational study. Int. J. Reprod. Contracept. Obstet. Gynecol. 7(4), 1523–1527 (2018).
Lü, L. et al. Effect of endometrial form and blood flow in the clinical outcome of frozen-thawed embryo transfer. Chin. J. Ultrasound. Med. 36(10), 930–933 (2020).
Lam, M. T., Li, H. W. R. & Ng, E. H. Y. Impact of endometrial thickness and volume compaction on the live birth rate following fresh embryo transfer of in vitro fertilization. J. Ultrasound. Med. 41(6), 1455–1463 (2022).
Maged, A. M. et al. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecol. Endocrinol. 35(11), 949–954 (2019).
Alcázar, J. L. Three-dimensional ultrasound assessment of endometrial receptivity: A review. Reprod. Biol. Endocrinol. 4(1), 1–13 (2006).
Xu, H. X., Yin, X. Y., Lu, M. D., Liu, G. J. & Xu, Z. F. Estimation of liver tumor volume using a three-dimensional ultrasound volumetric system. Ultrasound. Med. Biol. 29(6), 839–846 (2003).
Hidaka, H. et al. Reliability and validity of splenic volume measurement by 3-D ultrasound. Hepatol. Res. 40(10), 979–988 (2010).
Abuelghar, W. M. et al. Endometrial volume, and sub-endometrial blood flow indices as predictors of ICSI success. Gynecol. Obstet. Reprod. Med. 24(1), 27–33 (2018).
Nowak, P. M., Nardozza, L. M., Araujo Júnior, E., Rolo, L. C. & Moron, A. F. Comparison of placental volume in early pregnancy using multiplanar and VOCAL methods. Placenta. 29(3), 241–245 (2008).
Dogan, Y., Yucesoy, G., Ozkan, S. & Yucesoy, I. Three-dimensional volumetric study with VOCAL in normal and abnormal posterior fossa fetuses. J. Matern. Fetal Neonatal Med. 33(10), 1647–1655 (2020).
Becsek, A., Tzanidakis, N., Blanco, M. & Bollwein, H. Transrectal three-dimensional fetal volumetry and crown-rump length measurement during early gestation in mares: Intra- and inter-observer reliability and agreement. Theriogenology. 126, 266–271 (2019).
Avena-Zampieri, C. L. et al. Assessment of the fetal lungs in utero. Am. J. Obstet. Gynecol. MFM. 4(5), 100693 (2022).
Lian, X. et al. Reference range of fetal thorax using two-dimensional and three-dimensional ultrasound VOCAL technique and application in fetal thoracic malformations. BMC Med. Imaging. 21(1), 34 (2021).
ACOG Committee Opinion No. 734. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet. Gynecol. 131(5), e124–e129 (2018).
Khan, K. S. & Chien, P. F. Evaluation of a clinical test. I: Assessment of reliability. BJOG. 108(6), 562–567 (2001).
Raine-Fenning, N., Campbell, B., Collier, J., Brincat, M. & Johnson, I. The reproducibility of endometrial volume acquisition and measurement with the VOCAL-imaging program. Ultrasound. Obstet. Gynecol. 19(1), 69–75 (2002).
Yaman, C. et al. Reproducibility of three-dimensional ultrasound endometrial volume measurements in patients with postmenopausal bleeding. Ultrasound. Obstet. Gynecol. 19(3), 282–286 (2002).
Martins, W. P. et al. Reliability and validity of tissue volume measurement by three-dimensional ultrasound: An experimental model. Ultrasound. Obstet. Gynecol. 29(2), 210–214 (2007).
Yaman, C. & Mayer, R. Three-dimensional ultrasound as a predictor of pregnancy in patients undergoing ART. J. Turk. Ger. Gynecol. Assoc. 13(2), 128–134 (2012).
Funding
This project was supported by Guangdong High-level hospital construction fund (No. GD2019260), Sanming Project of Medicine in Shenzhen (No. SZSM202111011), Natural Science Foundation of Shenzhen Municipality (JCYJ20220530160209020), General Program for Clinical Research at Peking University Shenzhen Hospital (LCYJ2022001) and General Program for Basic Research at Peking University Shenzhen Hospital (JCYJ2021015).
Author information
Authors and Affiliations
Contributions
Y.W. made substantial contributions to the conception and design of the study, data acquisition, analysis and interpretation, and drafting and revising the manuscript. X.L., R.S., N.W., X.L., and Y.Z. contributed significantly to the literature review, data acquisition. H.W. provided guidance and supervision throughout the entire research project, and contributed significantly to the study design, interpretation of data, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Liu, X., Sun, R. et al. Automated endometrial identification and volume calculation in normal uteri using a novel smart ERA technique. Sci Rep 14, 20525 (2024). https://doi.org/10.1038/s41598-024-71069-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71069-z