Abstract
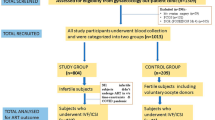
To analyze whether combinations of polymorphisms within FSHR gene influence ovarian response (OR) to stimulation. A multicenter prospective cohort study was conducted from 11/2016–06/2019 in Europe and Asia including predicted normo-responders under 38y. Patients underwent ovarian stimulation using fixed-dose 150 IU rFSH in a GnRH antagonist protocol. FSHR variants rs6165, rs6166 and rs1394205 were genotyped and combined in diplotypes. OR was compared following multivariable regression. rs6165/rs6166 genotype AG/AG exhibited more hypo-response (33.1% vs. 24%,adjOR 1.77 [95%CI 1.08–2.90]) and lower Follicle to Oocyte Index (FOI) compared with other diplotypes (EMD -11.72 [95%CI -20.89;-2.55]). Genotype GG/AA showed less hypo-response (19.1% vs. 31%, adjOR 0.48 [95%CI 0.24–0.96]), while AA/AA had higher FOI (EMD 20.04 [95%CI 4.51;35.56]). Concerning rs6165/rs1394205, less oocytes (EMD -1.99 [95%CI -3.57;-0.42]) and lower FOI (EMD -12.07 [95%CI -23.09;-1.05]) were retrieved with genotype AG/AG and higher FORT with genotype AA/AG (EMD 17.88 [95%CI 3.77;31.98]). Regarding rs6166/rs1394205, less hypo-response (16.3% vs. 29.5%,adjOR 0.42 [95%CI 0.19–0.97]), more oocytes (EMD 3.45 [95%CI 1.57;5.34]) and higher FOI (EMD 17.57 [95%CI 4.41;30.73) were found with genotype AA/GG. Genotype AA/AG presented higher FORT (EMD 13.47 [95%CI 2.51,24.42]), while more hypo-response (56.3% vs. 26.4%,adjOR 6.30 [95%CI 1.88;21.08]) and lower FOI (EMD -23.51 [95%CI -45.04;-1.97]) was reported with AG/AA. In accordance with our previous studies, FSHR polymorphisms have a statistically significant impact on OR, both individually and in association. However, only rs6166/rs1394205 genotype AA/GG seems to have a clinically significant effect, with a decrease in the prevalence of hypo-response, higher oocyte yield and increase in FOI.

Similar content being viewed by others
Data Availability
Data will be made available upon request.
Code Availability
Not applicable.
References
Neves AR, Montoya-Botero P, Sachs-Guedj N, Polyzos NP. Association between the number of oocytes and cumulative live birth rate: A systematic review. Best Pract Res Clin Obstet Gynaecol [Internet]. 2023;87:102307. https://doi.org/10.1016/j.bpobgyn.2022.102307.
Polyzos N, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril. 2018;110:661-670.e1.
Devesa M, Tur R, Rodríguez I, Coroleu B, Martínez F, Polyzos NP. Cumulative live birth rates and number of oocytes retrieved in women of advanced age. A single centre analysis including 4500 women ≥38 years old. Hum Reprod. 2018;33:2010–7.
Drakopoulos P, Blockeel C, Stoop D, Camus M, De Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31:370–6.
Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: An individual patient data approach. Hum Reprod Update. 2013;19:26–36.
Polyzos NP, Stoop D, Blockeel C, Adriaensen P, Platteau P, Anckaert E, et al. Anti-Müllerian hormone for the assessment of ovarian response in GnRH-antagonist-treated oocyte donors. Reprod Biomed Online. 2012;24:532–9.
Drakopoulos P, van de Vijver A, Parra J, Anckaert E, Schiettecatte J, Blockeel C, et al. Serum Anti-Müllerian Hormone Is Significantly Altered by Downregulation With Daily Gonadotropin-Releasing Hormone Agonist: A Prospective Cohort Study. Front Endocrinol (Lausanne). 2019;10:1–7.
Vaiarelli A, Drakopoulos P, Blockeel C, De Vos M, Van De Vijver A, Camus M, et al. Limited ability of circulating anti-Müllerian hormone to predict dominant follicular recruitment in PCOS women treated with clomiphene citrate: A comparison of two different assays. Gynecol Endocrinol. 2016;32:227–30.
Polyzos NP, Tournaye H, Guzman L, Camus M, Nelson SM. Predictors of ovarian response in women treated with corifollitropin alfa for in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2013;100:430–7.
Neves AR, Blockeel C, Griesinger G, Garcia-Velasco JA, La Marca A, Rodriguez I, et al. The performance of the Elecsys® anti-Müllerian hormone assay in predicting extremes of ovarian response to corifollitropin alfa. Reprod Biomed Online [Internet]. 2020;41:29–36. https://doi.org/10.1016/j.rbmo.2020.03.023.
Chinta P, Antonisamy B, Mangalaraj AM, Kunjummen AT, Kamath MS. POSEIDON classification and the proposed treatment options for groups 1 and 2: time to revisit? A retrospective analysis of 1425 ART cycles. Hum Reprod Open. 2021;2021:1–10.
Riccetti L, De Pascali F, Gilioli L, Santi D, Brigante G, Simoni M, et al. Genetics of gonadotropins and their receptors as markers of ovarian reserve and response in controlled ovarian stimulation. Best Pract Res Clin Obstet Gynaecol [Internet]. 2017;44:15–25. https://doi.org/10.1016/j.bpobgyn.2017.04.002.
Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: A systematic review and meta-analysis. Hum Reprod Update. 2018;24:1–16.
Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, et al. Mutation of the follicle-stimulating hormone receptor gene 5′-untranslated region associated with female hypertension. Hypertension. 2006;48:512–8.
Simoni M, Casarini L. Mechanisms in endocrinology: Genetics of FSH action: a 2014-and-beyond view. Eur J Endocrinol. 2014;170:R91-107.
Conforti A, Vaiarelli A, Cimadomo D, Bagnulo F, Peluso S, Carbone L, et al. Pharmacogenetics of FSH action in the female. Front Endocrinol (Lausanne). 2019;10:1–7.
Casarini L, Moriondo V, Marino M, Adversi F, Capodanno F, Grisolia C, et al. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol Cell Endocrinol [Internet]. 2014;393:83–91. https://doi.org/10.1016/j.mce.2014.06.013.
Santi D, Potì F, Simoni M, Casarini L. Pharmacogenetics of G-protein-coupled receptors variants: FSH receptor and infertility treatment. Best Pract Res Clin Endocrinol Metab [Internet]. 2018;32:189–200. https://doi.org/10.1016/j.beem.2018.01.001.
Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85:3365–9.
Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwaßer P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: A pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15:451–6.
Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I, et al. In Estimated Good Prognosis Patients Could Unexpected “hyporesponse” to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor? Reprod Sci. 2016;23:1103–8.
Huang X, Li L, Hong L, Zhou W, Shi H, Zhang H, et al. The Ser680Asn polymorphism in the follicle-stimulating hormone receptor gene is associated with the ovarian response in controlled ovarian hyperstimulation. Clin Endocrinol (Oxf). 2015;82:577–83.
Klinkert ER, te Velde ER, Weima S, van Zandvoort PM, Hanssen RGJM, Nilsson PR, et al. FSH receptor genotype is associated with pregnancy but not with ovarian response in IVF. Reprod Biomed Online [Internet]. 2006;13:687–95. https://doi.org/10.1016/S1472-6483(10)60660-8.
Laven JSE, Mulders AGMGJ, Suryandari DA, Gromoll J, Nieschlag E, Fauser BCJM, et al. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil Steril. 2003;80:986–92.
Mohiyiddeen L, Newman WG, McBurney H, Mulugeta B, Roberts SA, Nardo LG. Follicle-stimulating hormone receptor gene polymorphisms are not associated with ovarian reserve markers. Fertil Steril [Internet]. 2012;97:677–81. https://doi.org/10.1016/j.fertnstert.2011.12.040.
Genro VK, Matte U, De Conto E, Cunha-Filho JS, Fanchin R. Frequent polymorphisms of FSH receptor do not influence antral follicle responsiveness to follicle-stimulating hormone administration as assessed by the Follicular Output RaTe (FORT). J Assist Reprod Genet. 2012;29:657–63.
Tohlob D, Abo Hashem E, Ghareeb N, Ghanem M, Elfarahaty R, Byers H, et al. Association of a promoter polymorphism in FSHR with ovarian reserve and response to ovarian stimulation in women undergoing assisted reproductive treatment. Reprod Biomed Online [Internet]. 2016;33:391–7. https://doi.org/10.1016/j.rbmo.2016.06.001.
Neves AR, Vuong NL, Blockeel C, Garcia S, Alviggi C, Spits C, et al. The effect of polymorphisms in FSHR gene on late follicular phase progesterone and estradiol serum levels in predicted normoresponders. Hum Reprod. 2022;37:2646–54.
Polyzos NP, Neves AR, Drakopoulos P, Spits C, Alvaro Mercadal B, Garcia S, et al. The effect of polymorphisms in FSHR and FSHB genes on ovarian response: a prospective multicenter multinational study in Europe and Asia. Hum Reprod. 2021;36:1711–21.
Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602–18.
Montoya-Botero P, Martinez F, Rodríguez-Purata J, Rodríguez I, Coroleu B, Polyzos N. The effect of type of oral contraceptive pill and duration of use on fresh and cumulative live birth rates in IVF/ICSI cycles. Hum Reprod. 2020;35:826–36.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30:2005–8.
Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R. Serum anti-Mullerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod. 2011;26:671–7.
Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-to-oocyte (FOI) index. Front Endocrinol (Lausanne). 2018;9:1–7.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing [Internet]. Vienna, Austria; 2019. Available from: https://www.r-project.org/
Baldini GM, Catino A, Palini S, Sciorio R, Ferri D, Vinciguerra M, et al. The Polymorphism Asn680Ser on the FSH Receptor and Abnormal Ovarian Response in Patients with Normal Values of AMH and AFC. Int J Mol Sci. 2023;24:1080.
De Castro F, Ruiz R, Montoro L, Pérez-Hernández D, Sánchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80:571–6.
Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotropin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. 2009;18:509–15.
Lazaros L, Hatzi E, Xita NV, Takenaka A, Sofikitis N, Zikopoulos K, et al. Influence of FSHR Diplotypes on Ovarian Response to Standard Gonadotropin Stimulation for IVF/ICSI. J Reprod Med. 2013;58:395–401.
Sudo S, Kudo M, Wada SI, Sato O, Hsueh AJW, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8:893–9.
Yan Y, Gong Z, Zhang L, Li Y, Li X, Zhu L, et al. Association of follicle-stimulating hormone receptor polymorphisms with ovarian response in chinese women: A prospective clinical study. PLoS ONE. 2013;8:1–8.
Wunsch A, Ahda Y, Banaz-Yaşar F, Sonntag B, Nieschlag E, Simoni M, et al. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil Steril. 2005;84:446–53.
Desai SS, Achrekar SK, Paranjape SR, Desai SK, Mangoli VS, Mahale SD. Association of allelic combinations of FSHR gene polymorphisms with ovarian response. Reprod Biomed Online [Internet]. 2013;27:400–6. https://doi.org/10.1016/j.rbmo.2013.07.007.
Allegra A, Marino A, Raimondo S, Maiorana A, Gullo S, Scaglione P, et al. The carriers of the A/G-G/G allelic combination of the c.2039 A>G and c.-29 G>A FSH receptor polymorphisms retrieve the highest number of oocytes in IVF/ICSI cycles. J Assist Reprod Genet [Internet]. 2017;34:263–73. https://doi.org/10.1007/s10815-016-0835-9.
Paschalidou C, Anagnostou E, Mavrogianni D, Raouasnte R, Klimis N, Drakakis P, et al. The effects of follicle-stimulating hormone receptor (FSHR) -29 and Ser680Asn polymorphisms in IVF/ICSI. Horm Mol Biol Clin Investig. 2020;41:20190058.
Esteves SC, Yarali H, Vuong LN, Conforti A, Humaidan P, Alviggi C. POSEIDON groups and their distinct reproductive outcomes: Effectiveness and cost-effectiveness insights from real-world data research. Best Pract Res Clin Obstet Gynaecol [Internet]. 2022;85:159–87. https://doi.org/10.1016/j.bpobgyn.2022.05.003.
Acknowledgements
This study was supported by an unrestricted grant by Merck Sharp & Dohme (MSD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The Ethics Committee of the involved hospitals approved the study.
Consent to Participate
All participants gave their written informed consent for blood sampling and genetic investigations.
Consent for Publication
Not applicable.
Conflict of Interests/Competing Interests
C.B discloses honoraria for lectures from Abbott, Ferring, Organon, Merck, IBSA and Cook. LNV discloses grants from Merck Sharpe and Dohme as well as speaker and conference fees from Merck, Merck Sharpe and Dohme and Ferring and participation on a scientific board from Ferring. NPP reports grants from Merck Serono, Organon, Ferring Pharmaceutical, Roche Diagnostics, Theramex, IBSA, Gedeon Richter and Besins Healthcare; consulting fees from Merck Serono, Besins Healthcare, Organon and IBSA; honoraria from Merck Serono, Theramex, IBSA, Organon, Gedeon Richter, Besins Healthcare, Ferring Pharmaceuticals and Roche Diagnostics. ARN, SG and CS have no conflict of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43032_2024_1700_MOESM2_ESM.pdf
Supplementary file2 Linear regression models regarding the impact of FSHR diplotypes on the number of oocytes retrieved (PDF 415 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neves, A.R., Garcia, S., Vuong, L.N. et al. The Additive Effect of Combinations of FSH Receptor Gene Variants in Ovarian Response to Stimulation. Reprod. Sci. 31, 3560–3568 (2024). https://doi.org/10.1007/s43032-024-01700-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-024-01700-x