Abstract
To improve the quality of in vitro produced (IVP) embryos and reduce pregnancy losses, we proposed to reduce the components of the synthetic oviduct fluid (SOF) medium by 0% (SOF100), 50% (SOF50), and 75% (SOF25). First, embryos produced under these three treatments were evaluated for production, quality, lipid content, gene expression, and methylation patterns. The results indicated that all parameters analyzed were similar across all treatments (P > 0.05), suggesting that reducing media components does not affect embryo development and quality. Subsequently, we selected SOF25 for comparison with SOF100 in a commercial laboratory setting, evaluating embryo production, response to cryopreservation, gestation rate, and offspring birth. The data demonstrated that a 75% reduction in SOF medium components did not affect embryo development, quality, pregnancy rate, embryonic losses between 30 and 60 days, or birth rate (P > 0.05). To our knowledge, this is the first report on the pregnancy and birth rates of bovine blastocysts produced in media with nutrient concentrations as low as 25%. These results introduce novel cultural conditions that can be immediately incorporated into the IVF routine.
Similar content being viewed by others
Introduction
In vitro embryo production (IVP) is widely used in both human and livestock species, yet its overall efficiency remains suboptimal1,2. To enhance IVP procedures and the competence of the resulting embryos, significant attention has been directed towards optimizing the culture environment. Numerous studies have attempted to improve in vitro embryo development by manipulating the chemical environment, including the addition of various growth factors and3,4,5 supplements such as antioxidants6,7,8. However, the improvements achieved by adding different supplements have been limited, and embryo production rates have remained stable over the last decade. Additionally, the majority of studies aiming to improve IVP efficiency have focused on analyzing parameters such as embryo development rates, gene expression, and methylation status to indirectly infer IVP efficiency. These analyses are no longer sufficient; more robust and direct assessments, such as pregnancy and birth rates, are necessary if changes in the culture system are to be incorporated into commercial routines.
Recent studies have suggested that embryos might be exposed to an excess of nutrients in the culture medium, which could be detrimental to embryonic viability. Metabolomics studies have shown that the uptake of nutrients by embryos is significantly less than what is supplied in traditional culture media9,10,11,12, since embryos consume only a small proportion of the nutrients available in culture medium11. In addition, Herrick et al.13 found that reducing nutrient concentrations in the culture medium by half (50% dilution) did not affect developmental kinetics, blastocyst formation, hatching, cell allocation to the embryo transfer and inner cell mass, or adenosine triphosphate content in mouse embryos. Similarly, Herrick et al.9 demonstrated that bovine blastocysts produced with 25% nutrient concentrations could resist vitrification and warming as effectively as those produced with 100% nutrients. These authors showed that blastocyst formation was unaffected until nutrient concentrations were reduced to 6.25% of the total present in control conditions9. Furthermore, transcriptomic analysis of bovine blastocysts produced in vitro in conventional and reduced nutrient culture media revealed increased cellular signaling and membrane transport in those cultured in a reduced nutrient medium, suggesting these mechanisms may contribute to improved blastocyst development14.
Considering these results, we can infer that embryos utilize far fewer metabolites than what is available in the culture medium, indicating that the nutrient content in a wide variety of media far exceeds the embryo’s requirements. Based on this assumption, we hypothesized that reducing nutrients in the culture medium would improve embryo quality, resulting in higher pregnancy and birth rates. Since the majority of bovine commercial and research IVP laboratories prepare their culture media in-house, with synthetic oviduct fluid (SOF) being the most commonly used culture medium system15, we propose to reduce the SOF energetic components and evaluate how this reduction affects embryonic development, embryo quality, and the embryo’s ability to establish and maintain pregnancy.
Materials and methods
All procedures and protocols were performed following the Ethical Principles in Animal Research set forth by the Brazilian College of Animal Experimentation, with approval from applied by the Experimentation and Animal Use Committee (CEUA) of Embrapa Genetic Resource and Biotechnology, according to Brazilian laws for animal ethics and health research, under the protocol (005/2022) and in accordance with ARRIVE guidelines.
Chemicals and reagents
All in vitro production chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Oocyte collection, in vitro fertilization and embryo culture
Ovaries from crossbreed females (Bos taurus x Bos indicus) were collected immediately after slaughter and transported to the laboratory. Cumulus oocyte complexes (COCs) were aspirated from 3 to 8 mm diameter follicles. Only those with homogeneous cytoplasm and at least three layers of cumulus cells were used. After selection, approximately 25–30 COCs were transferred to 150 µL drops of in vitro maturation (IVM) media comprising tissue culture medium (TCM) 199 Earl’s salts (Invitrogen®—Thermo Fisher, Walthan, Massachusetts, USA), supplemented with 10% fetal bovine serum (FCS, Invitrogen®), 0.01 IU/mL follicle -stimulating hormone (FSH), 0.1 mg/mL L-glutamine, 1 µM cysteamine, 1 µM sodium pyruvate, and 0.075 mg/mL amikacin sulfate, and were cultured for 22 h at 38.5 °C. Frozen semen from a Nellore bull was used for in vitro fertilization (IVF). Motile spermatozoa were obtained using the Percoll (GE Healthcare, Piscataway, NJ, USA) method16 and added to the fertilization drop at a final concentration of 1 × 106 spermatozoa/mL. Spermatozoa and oocytes were co-incubated for 18 h at 38.5 °C with 5% CO2 in the air. The fertilization medium consisted of Tyrode’s albumin lactate pyruvate (TALP) supplemented with 2 mM penicillamine, 1 mM hypotaurine, 250 mM epinephrine, and 10 mg/mL heparin17.
After the 18-h co-incubation period, presumptive zygotes were washed and transferred to 150 µL droplets of synthetic oviductal fluid (SOF) medium18 supplemented with 50X essential and 100× non-essential amino acids, 0.34 mM sodium tricitrate, 2.77 mM myo-inositol, and 2.5% FCS (Invitrogen™, Waltham, MA, USA). For treatment groups, SOF medium was used with 100% (SOF100), 50% (SOF50), or 25% (SOF25) of carbohydrates and amino acids (Supplementary Table 1). For all groups, embryos were cultured at 38.5 °C with 5% CO2 and were evaluated on D2 for cleavage and on D6, 7, and 8 for blastocyst development.
Lipids quantification
Staining and lipid measurements in day 7 expanded blastocyst were performed as previously described by Faria et al.19. Briefly, embryos were fixed and stained with Bodipy 493/503 (Molecular Probes; 20 µg/mL). After staining, embryos were evaluated using a confocal microscope (LSM Leica Sp8 Laser Dissection Microscope, New Orleans, LA, USA). All samples were analyzed and photographed, and the images were processed using ImageJ software (National Institutes of Health). The background was corrected, and lipid quantification was based on the ratio of the area occupied by lipids in the cytoplasm to the total area of the oocyte.
DNA methylation
Expanded blastocyst (D8 of development) were used to analyze the DNA methylation of two loci—B. taurus bovine testis satellite I (Satellite I) and B. taurus alpha satellite I DNA (α-Satellite). Three pools of five embryos each were used for methylation analysis. Table 1 shows the primer sequences, GenBank accession numbers, amplicon lengths, and CpG numbers. Pooled embryos were incubated with pronase E at a concentration of 10 mg/mL to digest the zona pellucida at 37 °C for 30 min and at 85 °C for 15 min to inactivate the enzyme. DNA was extracted using thermal shock, where samples were frozen in liquid nitrogen and immediately placed in a thermocycler at 95 °C for 1 min, repeated five times. DNA samples were treated with sodium bisulfite using the EZ DNA Methylation-Lightning™ Kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions. Bisulfite-treated DNA was subjected to PCR under the following conditions: a total volume of 20 µL comprising 1× Taq buffer, 1.5 mM MgCl2, 0.4 mM dNTPs, 1 U Platinum™ Taq polymerase (Invitrogen, CA, USA), 0.5 µM of each primer (forward and reverse), and 3 µL of bisulfite-treated DNA. Polymerase Chain Reaction (PCR) cycling was carried out with an initial denaturing step at 94 °C for 3 min, followed by 40 cycles at 94 °C for 40 s, 45 °C for 1 min, and 72 °C for 1 min, with a final extension at 72 °C for 15 min. Amplicons were purified from agarose gel using the Wizard SV Genomic DNA Purification System (Promega Corporation, Madison, WI, USA), according to the manufacturer’s instructions. Purified amplicons were cloned into the TOPO TA Cloning vector (PCRII-TOPO® vector system, Invitrogen, Carlsbad, CA, USA) and transferred into DH5α cells using a heat shock protocol. Plasmid DNA was isolated using the QIprep Spin Miniprep Kit (Qiagen), and individual clones were sequenced using BigDye® cycle sequencing chemistry and an ABI3100 automated sequencer.
The sequencing quality was analyzed using Chromas® and the methylation patterns were quantified with the Quantification tool for Methylation Analysis (QUMA, http://quma.cdb.riken.jp/top/index.html)20. DNA sequences were compared with GenBank reference sequences (accession numbers shown in Table 1). Only sequences originating from clones with ≥ 95% identity and cytosine conversion were used. The conversion rate of non-CpG cytosines was used to calculate the efficiency of bisulfite treatment, and the methylation pattern was used to identify individual clones from different DNA templates21.
RT-qPCR
The relative abundance of transcripts for six target genes related to lipid metabolism (SLC2A1, SLC2A3, PLIN 2, CPT2, ELOVL1, FABP3) was quantified by RT-qPCR.
D7 expanded blastocysts were selected, placed in RNAlater Stabilization Solution (Ambion®, Thermo Fisher, Walthan, Massachusetts, USA), and stored at − 80 °C until further processing. Total RNA was isolated from three pools of 12 embryos from each treatment using the RNeasy Plus Micro Kit (Qiagen®, Hilden, Germany), following the manufacturer’s instructions. The entire volume of each RNA sample was used for the synthesis of complementary DNA (cDNA) using the GoScript Reverse Transcriptase Kit (Promega®, Wisconsin, USA) with Oligo-dT (0.5 µg/µL) and Random (0.5 µg/µL) primers. The reactions were incubated at 65 °C for 5 min, 50 °C for 50 min, and 85 °C for 5 min. qPCR was carried out using the GoTaq qPCR Master Mix Kit (Promega®), according to the manufacturer’s instructions in a7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Each sample was analyzed in triplicates. The specificity of each amplicon was determined by melting curve analysis and checking the amplicon size on agarose gel. Reactions were performed with cDNA equivalent to 0.6 embryos. qPCR cycling conditions were 95 °C for 5 min, followed by 50 cycles of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 30 s. Table 2 shows gene nomenclature, primer sequences and concentrations, GenBank accession number, amplicon length, and the efficiency of each primer pair. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene for data normalization22. Relative gene expression was calculated using the ΔΔCt method, with efficiency correction as described by Pfaffl21.
Slow freezing and thawing of the embryos
Embryos were cryopreserved using the slow freezing method. Briefly, 111 expanded blastocysts (grade I, according to IETS manual23) were exposed to VIGRO® Ethylene Glycol Freeze Plus with Sucrose media (Vetoquinol, Lavaltrie, Canada) at room temperature and individually loaded into 0.25 mL straws (Minitube). After 10 min, the straws were placed in an embryo slow -freezing machine (TK-1000, TK Tecnologia, Brazil) with a cryopreservation curve starting at − 6 °C, seeding after 2 min at this temperature, decreasing the temperature at a rate of 0.5 °C/min until − 35 °C, and holding for 10 min at this temperature. After the holding time, the straws were submerged and stored in liquid nitrogen.
For thawing, the straws were removed from liquid nitrogen and held at room temperature for 10 s, followed by submersion in water at 33–35 °C for 30 s. The embryos were then unloaded onto Petri dishes and placed in 100 µL of their respective SOF medium under mineral oil. They were kept under the same in vitro culture (IVC) conditions for 48 h to evaluate survival and hatching rates.
Embryo transfer and pregnancy diagnosis
Crossbred heifers and cows (Bos indicus x Bos taurus) with a body condition score (BCS) between 2.5 and 3.5 were used as recipients. On a random day of the estrous cycle, the selected recipients underwent progesterone and estradiol benzoate-based hormone synchronization to induce estrus on the same day as the IVF (D0). On the day of embryo transfer, all recipients were evaluated via ultrasound. Recipients with a corpus luteum (CL) in one of the ovaries were considered suitable to receive an embryo. Fresh embryos, grade I expanded blastocyst stage on day 7 of development were transferred into the ipsilateral uterine horn of the ovary with the CL. Pregnancy diagnoses were performed via ultrasonography at 30 days (D30) and 60 days (D60) post-transfer.
Experimental design
The present study aimed to investigate whether reducing nutrient availability in the culture medium would benefit embryo development and quality. The study comprised three sets of experiments structured as follows:
Experiment 1: effect of reducing the components of the SOF culture medium on the production and quality of embryos
The impact of reducing components in the SOF medium, used for embryo culture, on both the quantity and quality of in vitro produced embryos was evaluated in a seven replicates experiment. Three treatments were employed:
-
Control (SOF100) Embryos cultured in SOF medium supplemented with 2.5% bovine fetal serum (FCS).
-
SOF50 Embryos cultured in SOF medium with a 50% reduction in components, supplemented with 2.5% FCS.
-
SOF25 Embryos cultured in SOF medium with a 75% reduction in components, supplemented with 2.5% FCS.
After IVF presumptive zygotes were randomly assigned to these three groups and cultured for 8 days. Embryo development was assessed for cleavage on D2 and blastocyst formation rates on D6, D7, and D8. On Day 7, embryos were subjected to lipid quantification and gene expression analyses. Bodipy dye was used for staining, and confocal microscopy was employed for image analysis. A total of 20 embryos for SOF100, 22 for SOF50, and 17 for SOF25 were used at the blastocyst stage. For quantification of transcripts related to lipid metabolism, 3 pools of 12 expanded blastocysts were used. Additionally, 3 pools of 5 expanded blastocysts on day 8 of development were used to evaluate DNA methylation patterns of repetitive DNA regions: α-Satellite and Satellite-1, selected for their multiple copies in the genome, indicating broader genome methylation patterns.
Experiment 2: the commercial use of reduced components SOF culture medium: effects on embryo production and cryotolerance
Based on the findings of Experiment 1, the treatment using SOF medium with only 25% of the total components was selected due to yielding similar results to the control. Experiment 2 was conducted at a commercial IVF laboratory (Embriotec Reprodução Animal, Anápolis, GO, Brazil) to validate the feasibility of using the reduced medium in commercial settings and to assess its impact on embryo production, survival, and hatching post-freezing.
All procedures for IVM, IVF, and IVC were consistent with those used in Experiment 1, except for the number of treatments (SOF100 and SOF25) and the day of cleavage evaluation. A total of 2485 COCs across 7 replicates were assigned to one of the aforementioned treatment groups. Embryo development was monitored with cleavage evaluated on D4 and blastocyst production on D7 and D8 post-fertilization. On D7 only grade I expanded blastocysts (n = 111) were subjected to cryopreservation using the slow freezing method. Post-thawing, embryos were transferred to 100 µL of their respective SOF medium and maintained under identical conditions for 48 h to assess survival through expansion and hatching rates.
Experiment 3: the commercial use of reduced components SOF culture medium: effects on pregnancy, embryonic losses, and the birth rates
Experiment 3 mirrored Experiment 2 but with a focus on evaluating the reproductive outcomes of embryos produced in SOF100 and SOF25 cultures. Expanded blastocysts from both groups at D7 of development were transferred to previously synchronized recipient animals. A total of 1539 viable COCs were recovered from 92 Gyr donors and fertilized with female sex-sorted semen from six Holstein bulls. The resulting presumptive zygotes were divided into two treatment groups (SOF100: 851 COC and SOF25: 688 COC). At D7 grade I expanded blastocysts were transferred into the ipsilateral uterine horn of recipients with a CL (SOF100: 280; SOF25: 174). Pregnancy was monitored via ultrasonography at D30 and D60. Pregnant recipients were observed daily until the expected calving time to record pregnancy outcomes and the delivery of offspring.
Statistical analysis
Statistical analyses were conducted using Prophet Program, version 5.0 (1997), GraphPad Prism (https://www.graphpad.com/scientific-software/prism), or SAS (SAS Studio 3.8, University Edition; SAS Institute Inc., Cary, NC, USA) software, with significance set at P-values ≤ 0.05. Blastocyst rates and developmental kinetics of IVP embryos were analyzed using Chi-square tests. Gene expression and DNA methylation data were compared among experimental groups using analysis of variance (ANOVA) and Tukey’s test or Kruskal–Wallis and Mann–Whitney tests for normally and non-normally distributed data, respectively. Lipid quantification data were analyzed by ANOVA using the SAS Proc Glimmix model (SAS Studio 3.8, University Edition; SAS Institute Inc., Cary, NC, USA). Differences between media were assessed using Tukey’s test.
Results
Effect of reducing the components of the SOF culture medium on the production and quality of embryos
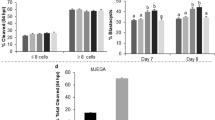
Embryo production using media with a 50% or 75% reduction in components is presented in Table 3. A beneficial effect of the component reduction can be observed at D7 of development in the SOF25 group. However, embryo developmental kinetics were similar among all groups (P > 0.05) (Table 4).
Embryos produced in different culture media were also evaluated for lipid accumulation and the expression of genes related to lipid metabolism. A total of 81 embryos were evaluated, and the results showed that the average area occupied by lipids did not significantly differ among embryos from all groups (Fig. 1), indicating no effect of the culture systems on lipid accumulation.
Regarding gene expression, no effect of reducing medium components on transcript quantification was observed (Fig. 2). These data align with the phenotypic observation of lipid accumulation in embryos as verified by Bodipy staining, which also showed similar results (Fig. 1).
Relative abundance of mRNA encoding the SLC2A1, SLC2A3, PLIN2 2, CPT2, ELOVL, and FABP3 genes determined by qPCR in embryos produced in SOF medium [Control (SOF100)], in SOF medium in which the concentration of components was reduced by 50% (SOF50) and 75% (SOF25). Mean ± standard deviation of the mean (SD) of four biological replicates. ANOVA, significance level P < 0.05.
To assess whether the reduction in components would affect the methylation pattern of embryos, two regions of repetitive DNA (α-Satellite and Satellite-1) were compared. The methylation profiles of α-Satellite and Satellite-1 were similar across all groups (Fig. 3).
Methylation pattern in the α-Satellite and Satellite-I regions in expanded blastocysts produced in vitro. Embryos cultured in SOF100, SOF50 and SOF25, were analyzed. Each line represents a clone, and each circle represents a CpG. White circles are demethylated CpGs and black circles are methylated CpGs. Data were compared among experimental groups using the Kruskal–Wallis test or Mann–Whitney test, considering P values ≤ 0.05 as statistically significant.
The commercial use of reduced components SOF culture medium: effects on embryo production and cryotolerance
Based on the results of Experiment 1, a medium containing 25% of the components was chosen to be tested in a private laboratory to confirm our findings and evaluate pregnancy and birth rates.
Initially, the in vitro production of embryos was tested in routine laboratory conditions by comparing the SOF medium with only 25% of the components with the control 100% SOF medium. Results showed that embryo production was similar for both groups (Supplementary Table 2). Similarly, no effect of the medium was observed on the embryos’ response to cryopreservation, as both survival and hatching rates were comparable between treatments (Supplementary Tables 3 and 4).
The commercial use of reduced components SOF culture medium: effects on pregnancy, embryonic losses, and birth rates
Finally, embryos produced in SOF100 and SOF25 were transferred to previously synchronized recipients, and pregnancy rates were assessed. The 75% reduction in medium components did not affect pregnancy rates at 30 and 60 days, embryonic losses between 30 and 60 days, nor birth rates (Table 5).
Discussion
In vitro culture techniques have significantly advanced assisted reproductive technologies; however, their potential limitations and drawbacks on embryo quality and developmental outcomes must be acknowledged. Efforts to optimize in vitro conditions persist to enhance embryo quality and increase success rates. Despite ongoing research, embryo production and loss rates have shown stability over the past decade. Recent metabolic studies have suggested that excessive nutrients in culture media might adversely affect embryonic viability. Building on this evidence, we hypothesized that reducing culture medium components would not compromise embryo production or the ability to establish and maintain pregnancy.
In our first experiment, we reduced by 50% and 75% the energetic components of the SOF medium, from what is commonly used in commercial laboratories for bovine embryo production. Surprisingly, our results indicated that this reduction did not impair embryo production; instead, the group with a 75% reduction showed higher embryo yields compared to other groups. Similar findings of reduced media components not negatively impacting embryo development have been reported in the literature9,12,24. For instance, in mouse embryos, reducing nutrient concentrations by 50% did not affect developmental kinetics, blastocyst formation, hatching, or cell allocation to the TE and ICM. However, a 75% reduction delayed development, reduced overall blastocyst formation and hampered hatching24. Interestingly, these effects were not observed in bovine embryos until nutrient concentrations were reduced to 6.25%9. Our findings align with these reports, confirming that reductions of up to 75% in nutrients, specifically amino acids and carbohydrates, do not affect bovine embryo production rates. Previous studies have indicated that most culture media, including SOF used in our study, contain concentrations of carbohydrates and amino acids akin to the oviduct and uterine fluid, suggesting a physiological similarity that may benefit embryos. However, the non-static environment and smaller fluid volumes in culture drops could potentially limit nutrient availability to embryos compared to their natural conditions. It is plausible that embryos require less than what is typically available in culture medium drops, suggesting they may need fewer nutrients than are provided in standard media24. This could explain why reducing nutrient concentrations did not adversely affect embryo development as observed in our study. These insights highlight the complexity of optimizing culture conditions to better mimic physiological environments while ensuring optimal embryo development. Future studies could explore fine-tuning nutrient concentrations or supplementing with metabolic factors to further enhance embryo quality and developmental outcomes in assisted reproduction technologies.
Although embryo development appeared not be affected by culture in reduced nutrient conditions, we evaluated whether this could impact embryo quality. The first evaluation focused on the kinetic of embryo development. No differences were found in developmental speed; both groups showed similar percentages of expanded and hatched blastocysts by D7 of development. Considering that embryos developing earlier are more likely to establish a pregnancy1,25,26, it may be assumed that, for this parameter, the embryos had similar quality.
In contrast, studies using a more extreme nutrient reduction (6.25%) reported lower total cell numbers in blastocysts, suggesting a potential impact on cellular quality27. However, upregulation of PLAC8, a gene associated with embryo quality, indicated that these embryos might still perform well post-transfer despite the reduction in cell numbers. Unlike these findings, our study showed no differences in developmental speed or gene expression, which could be attributed to the less drastic nutrient reduction in our culture medium (25% instead of 6.25%). The more moderate reduction in our medium might have provided a better balance of metabolic support than the conditions with extremely limited nutrients. The more moderate reduction in our medium might have provided a better balance of metabolic support than the conditions with extremely limited nutrients. An extreme reduction could potentially limit the availability of some essential components required by the embryo, which is highly plausible since previous studies have demonstrated that adding small amounts of pyruvate and lactate to extremely reduced media can rescue developmental competence27. Additionally, medium supplemented with l-carnitine and either lipid-free or lipid-rich BSA improved blastocyst development, with better outcomes observed in the reduced nutrient lipid-rich medium compared to the other two groups28.
Embryos were further evaluated for lipid accumulation and the expression of genes related to lipid metabolism. Mammalian embryos utilize pyruvate, fatty acids, and amino acids as energy sources28. We questioned whether reducing these components would affect lipid content in embryos. Increased lipid accumulation in in vitro embryos has been linked to developmental stress29, making it a potential indicator of embryo quality. Using a specific dye for neutral lipids, we found that the area occupied by lipids in embryos from all groups was similar (p > 0.05%) to that in the control medium SOF 100%. Gene expression analysis related to lipid metabolism showed no significant changes, confirming the phenotype of lipid accumulation observed in embryos stained with Bodipy.
To better understand the impact of nutrient reduction, we delved deeper into the analysis of methylation patterns in embryos. We compared the methylation status of two loci of repetitive DNA, α-Satellite and Satellite-I. These loci were chosen because of their widespread presence in the genome, which could reflect broader DNA methylation patterns30. Our results indicated that both loci investigated were hypomethylated (less than 11%), with no discernible differences in methylation patterns among treatments. Despite our expectation that reducing nutrient excess might improve embryo quality, the methylome, like other parameters, was not affected by the reduction of media components.
An analysis of the results indicates that the current SOF media contains nutrient levels significantly higher than what embryos actually require. Our findings show that reducing these nutrient components does not negatively impact either the production or quality of in vitro produced embryos. However, for these changes to be adopted in commercial settings, it was essential to confirm that they would not adversely affect embryos, even during advanced stages of development. To ensure this, we partnered with a commercial laboratory to assess pregnancy and birth rates, which are recognized as the gold standard for evaluating embryo viability. Notably, previous studies have not evaluated pregnancy and birth rates, making our work a novel contribution to the field by providing a more comprehensive understanding of the impact of nutrient levels on embryo success.
For this evaluation, we used only two treatments: SOF100 (control) and SOF25 (reduced). Initially, the reduced medium was tested for production and response to cryopreservation. The outcomes of IVF were similar to those found in the initial experiment. Concerning the response to cryopreservation, the SOF25 group initially had a lower hatching rate post-thawing, but within 48 h, hatching rates were comparable to SOF100. These results reinforce that reducing nutrient concentrations by 75% in the media composition does not compromise the production or quality of IVP embryos. The most critical data pertained to pregnancy, embryo loss, and birth rates. While other studies have shown that embryos can tolerate reduced nutrient concentrations9,11,12,13,27, to our knowledge, this is the first report of pregnancy and birth rates of bovine blastocysts produced in media containing as little as 25% of the usual nutrient concentrations. It is noteworthy that a considerable number of embryos were transferred in each group, supporting the reliability of these results.
In summary, this study demonstrates that bovine embryos produced in vitro exhibit high adaptability to variations in extracellular nutrient availability or that conventional culture media contain significantly more nutrients than embryos require. Whatever the underlying reason, it is evident that the SOF medium commonly used for IVP in cattle can have its nutrient concentrations reduced by 75% without compromising embryo viability. These findings propose novel culture conditions that can be immediately incorporated into IVF routines. Furthermore, they underscore the importance of further research to expand our understanding of embryo metabolism and contribute to the development of new culture media.
Data availability
The datasets generated during and/or analysed during the current study are provide within the manuscript or supplementary information files.
References
de Lacerda, I. P. et al. Cattle breed affects in vitro embryo production in a large-scale commercial program on dairy farms. Livest. Sci. 240, 104135 (2020).
Ealy, A. D., Wooldridge, L. K. & Mccoski, S. R. Board Invited Review: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci 97, 2555–2568 (2019).
Amaral, T. F. et al. Actions of CSF2 and DKK1 on bovine embryo development and pregnancy outcomes are affected by composition of embryo culture medium. Sci. Rep. 12, 7503 (2022).
Stoecklein, K. S., Sofia Ortega, M., Spate, L. D., Murphy, C. N. & Prather, R. S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 16, e0243727 (2021).
Seekford, Z. K. et al. Interleukin-6 supplementation improves post-transfer embryonic and fetal development of in vitro-produced bovine embryos. Theriogenology 170, 15–22 (2021).
Marques, T. C. et al. Blastocoel fluid removal and melatonin supplementation in the culture medium improve the viability of vitrified bovine embryos. Theriogenology 160, 134–141 (2021).
Fidelis, A. A. G. et al. Ethanolic extract of dried leaves from the cerrado biome increases the cryotolerance of bovine embryos produced in vitro. Oxid. Med. Cell. Longev. 2020, 1–16 (2020).
dos Santos Mendonça-Soares, A., Guimarães, A. L. S., Fidelis, A. A. G., Franco, M. M. & Dode, M. A. N. The use of insulin-transferrin-selenium (ITS), and folic acid on individual in vitro embryo culture systems in cattle. Theriogenology 184, 153–161 (2022).
Herrick, J. R. et al. Developmental and molecular response of bovine embryos to reduced nutrients in vitro. Reprod. Fertil. 1, 51–65 (2020).
Herrick, J. R., Greene, A. F., Becker, J., Schoolcraft, W. B. & Krisher, R. L. 82 How low can you go? Defining the minimal nutrient requirements for bovine embryos in culture. Reprod. Fertil. Dev. 29, 148–148 (2017).
Krisher, R. L. et al. Applying metabolomic analyses to the practice of embryology: Physiology, development and assisted reproductive technology. Reprod. Fertil. Dev. 27, 602–620 (2015).
dos Santos, É. C. et al. Less is more: Reduced nutrient concentration during in vitro culture improves embryo production rates and morphophysiology of bovine embryos. Theriogenology 173, 37–47 (2021).
Herrick, J. R. et al. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats and humans. Reprod. Fertil. Dev. 27, 323–331 (2015).
Ming, H. et al. In vitro culture alters cell lineage composition and cellular metabolism of bovine blastocyst. Biol. Reprod. https://doi.org/10.1093/BIOLRE/IOAE031 (2024).
Ferré, L. B. et al. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 14, 991–1004 (2020).
Machado, G. M. et al. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology 71, 1289–1297 (2009).
Parrish, J. J., Krogenaes, A. & Susko-Parrish, J. L. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 44, 859–869 (1995).
Holm, P., Booth, P. J., Schmidt, M. H., Greve, T. & Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 52, 683–700 (1999).
Faria, O. A. C. et al. Maturation system affects lipid accumulation in bovine oocytes. Reprod. Fertil. Dev. 33, 372–380 (2021).
Kumaki, Y., Oda, M., & Okano, M. QUMA: quantification tool for methylation analysis Nucleic Acids Research 36(Web Server) W170-W175. https://doi.org/10.1093/nar/gkn294 (2008).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Fleige, S. et al. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 28, 1601–1613 (2006).
The International Embryo Technology Society (IETS) > Meetings > 2025 IETS Annual Meeting. https://www.iets.org/Meetings/2025-IETS-Annual-Meeting.
Ermisch, A. F. et al. A novel culture medium with reduced nutrient concentrations supports the development and viability of mouse embryos. Sci. Rep. 10, 9263 (2020).
Pribenszky, C., Nilselid, A. M. & Montag, M. Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: A meta-analysis. Reprod. Biomed. Online 35, 511–520 (2017).
Florentino, C. et al. Pregnancy rates bovine recipients inovulated with in vitro produced (IVP) embryos in the legal amazon. J. Anim. Sci. Adv. 3, 193 (2013).
Pasquariello, R. et al. Lipid enriched reduced nutrient culture medium improves bovine blastocyst formation. Reprod. Fertil. 4, e230057 (2023).
Bradley, J. & Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 63, 93–103 (2019).
Melo-Sterza, F. A. & Poehland, R. Lipid metabolism in bovine oocytes and early embryos under in vivo, in vitro, and stress conditions. Int. J. Mol. Sci. 22, 3421 (2021).
Silveira, M. M. et al. DNA methylation profile at a satellite region is associated with aberrant placentation in cloned calves. Placenta 70, 25–33 (2018).
Funding
Grant support: Embrapa, project 30.20.90.022.00.00. FAPDF, project 00193-00002398/2023-67.
Author information
Authors and Affiliations
Contributions
LO Leme, data collection, analysis and interpretation of results, and manuscript preparation; MM Franco, study conception, analysis and interpretation of results and manuscript revision; OA Farias, data collection, analysis and interpretation of results; AR Caetano, analysis tools; JG Souza, data collection; LR Carvalheira, data collection; ES Filho, study conception and design, data collection, analysis and interpretation of results; MAN Dode, study conception and design, analysis and interpretation of results, and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
de Oliveira Leme, L., Franco, M.M., de Faria, O.A. et al. Reduction of nutrients concentration in culture medium has no effect on bovine embryo production, pregnancy and birth rates. Sci Rep 15, 4839 (2025). https://doi.org/10.1038/s41598-025-88864-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88864-x