Abstract
In couples with of non-male factor infertility, prevailing discussions have focused primarily on patients undergoing fresh embryo transfer. However, whether intracytoplasmic sperm injection (ICSI) improves reproductive outcomes in patients with non-male factor infertility undergoing frozen-thawed embryo transfer (FET) treatment remains unclear. This retrospective study analyzed 57,907 cycles from the Human Fertilisation and Embryology Authority. All FET cycles with non-male factor infertility were initially included. The final included cycles were divided into the ICSI and in vitro fertilization (IVF) groups. Primary outcomes include clinical pregnancy rate, live birth rate, and miscarriage rate; secondary outcomes comprised neonatal outcomes such as birthweight, gestational week, and sex. Binary logistic regression analysis was used to investigate the impact of ICSI on the studied population. The overall clinical pregnancy rate and live birth rate were significantly higher in the ICSI group than in the IVF group (29.6% vs. 26.0%, P < 0.001; 21.5% vs. 19.1%, P < 0.001). However, ICSI showed no significant association with clinical pregnancy or live birth [adjusted OR: 1.01 (0.95, 1.05), P = 0.969; 1.05 (0.86, 1.28), P = 0.611] after adjustment for confounders. Furthermore, while ICSI was associated with increased rates of full-term births and normal birthweight in singletons, these associations were attenuated after adjustment. Finally, ICSI exhibited no significant effect on neonatal sex ratio [adjusted OR: 0.91(0.93, 1.01), P = 0.052]. In conclusion, ICSI was not associated with improved clinical or neonatal outcomes in FET cycles with non-male factor infertility.
Similar content being viewed by others
Introduction
Intracytoplasmic sperm injection (ICSI) is an insemination method in which a single sperm is directly injected into an egg to achieve fertilization1. Initially developed for severe male factor infertility involving poor sperm quality or quantity2,3. ICSI is currently increasingly used in cases of non-male factor infertility, including previous failed conventional in vitro fertilization (IVF) cycles, unexplained infertility, or advanced maternal age. However, its efficacy and safety in these cases are still under investigation4,5. While some studies propose that ICSI enhances fertilization rates and embryo quality in these cases6, others report that ICSI does not improve pregnancy or live birth rates in these cases and may even increase the risk of birth defects7,8. The American Society for Reproductive Medicine (ASRM) guidelines emphasize insufficient evidence to universally recommend ICSI for non-male factor IVF cycles, advocating instead for individualized decision-making based on clinical context and patient preferences2.
Frozen-thawed embryo transfer (FET) is a treatment cycle in which cryopreserved embryos created in the oocyte pick-up cycle are thawed and transferred into a woman’s uterus9. FET offers multiple advantages, including higher success rates, scheduling flexibility, reduced ovarian hyperstimulation syndrome (OHSS) risk, multiple transfer opportunities, cost-effectiveness per live birth, and lower risk of preterm delivery, small for gestational age and low birth weight for singleton babies10,11,12,13,14.
In non-male factor infertility, prevailing discussions have focused primarily on patients undergoing fresh embryo transfer15,16,17. Notably, only the study by Li et al.7 has evaluated insemination method (ICSI vs. IVF) effects on embryos used in FET cycles, incorporating both fresh transfers and subsequent FETs. Insemination method may influence embryo quality, as fresh embryos generally exhibit higher implantation potential than frozen-thawed counterparts, irrespective of fertilization technique. However, cryopreservation processes may compromise embryo viability, with ICSI-inseminated embryos potentially showing marginally lower post-thaw survival rates compared to conventionally fertilized embryos7. To address this knowledge gap, this study aims to investigate the potential impact of insemination methods involving embryos transferred in FET cycles on the reproductive outcomes of couples with non-male factor infertility.
Methods
Study population and participants
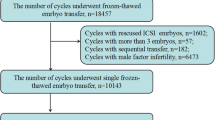
The anonymized dataset was obtained from the Human Fertilisation and Embryology Authority (HFEA) (www.hfea.gov.uk), which is freely accessible for public use and covers the period from 1995 to 2018. Ethical approval was waived as this study utilized de-identified secondary data. For the purpose of this study, only cycles with non-male factor infertility and FET treatment were initially included. The detailed inclusive procedure of cycles of interest is shown in Fig. 1. Specifically, cycles prior to 1995 were not considered for inclusion because of the extremely low number of ICSI uses in non-male factor infertility; cycles involving embryos subjected to biopsy and genetic testing were excluded; either cycles of transferred embryos developed from donor gametes or inseminated with unknown insemination methods were excluded; Finally, the included cycles were divided into two groups (ICSI vs. IVF) based on the insemination methods of transferred embryos.
Primary outcome measure
The primary outcomes were clinical outcomes such as the clinical pregnancy rate, miscarriage rate, live birth rate, and implantation rate. Clinical pregnancy was defined as the presence of one or more gestational sac(s) with evidence of foetal heart pulsations observed via ultrasound examination. Live birth was defined as the delivery of at least one live baby, which was recorded in the dataset. Miscarriage was defined as the loss of a clinical pregnancy that did not end up with termination, live birth or still birth (still birth: where a baby is born after 24 weeks gestation showing no signs of life). The implantation rate was calculated as the proportion of fetal sacs with fetal pulsation per transferred embryo. The pregnant women who were lost to follow-up were recorded as not experiencing any events. The clinical pregnancy rate and live birth rate were calculated per cycle of treatment, whereas the miscarriage rate was calculated per clinical pregnancy.
Secondary outcome measures
The secondary outcomes were neonatal outcomes, such as gestational age, birthweight, and sex ratio at birth, which were separately shown on the basis of the number of live births. Although the data are categorical variables, gestation weeks were further divided into three groups: very preterm birth (< 32 weeks), preterm birth (32–36 weeks), full-term birth (37–39 weeks), and post-term birth (≥ 40 weeks, which is different from the usual threshold of 42 weeks), and birth weight was divided into four groups: very low birth weight (< 1.5 kg), low birth weight (1.5–2.5 kg), normal birth weight (2.5–4.0 kg), and high birth weight (> 4.0 kg).
Statistical analyses
Because the characteristics are shown as categorical variables, the data are displayed as relative frequencies. Comparisons of the general characteristics and reproductive outcomes between the IVF group and the ICSI group were performed via the chi-square tests. Owing to the feature of the anonymous data, we were unable to link the cycles that belonged to the same couples. It was thus impossible to calculate the cumulative clinical pregnancy rate or cumulative live birth rate to investigate the impact of insemination methods on reproductive outcomes via the generalized estimation equation. However, univariable or multivariable logistic regression could be used to determine the effects of ICSI compared with those of IVF on reproductive outcomes. Given that neonatal outcomes significantly differ between singleton and twin live births, the birthweight and gestation times of the ICSI and IVF groups were compared separately. Statistical analyses were carried out via IBM SPSS Statistics 22.0 (Armonk, NY: IBM Corp.). P values < 0.05 were considered statistically significant for the analysis.
Results
Patient characteristics
As shown in Fig. 1, a total of 57,907 cycles from 1995 to 2018 were included in the final analysis, with 12,976 cycles (22.4%) ICSI-inseminated embryos. Although the ages of the females in the ICSI and IVF groups were similar, the number of previous treatment cycles significantly differed. The proportions of women who achieved successful clinical pregnancy and delivery through ART were significantly greater in the ICSI group. Furthermore, women in the ICSI group were more likely to be diagnosed with unexplained infertility but less likely with infertility with tubal disease and endometriosis. The ICSI group had a significantly greater proportion of cycles with the single embryo transfer strategy (Table 1).
Primary outcomes
The overall implantation rate, clinical pregnancy rate, and live birth rate were significantly greater in the ICSI group (19.9% vs. 15.8%, P < 0.001; 29.6% vs. 26.0%, P < 0.001; 21.5% vs. 19.1%, P < 0.001) than those in the IVF group, and the differences mostly remained consistent when subgroup analyses on the basis of stratified ages and numbers of transferred embryos were performed. For women without tubal disease, ovulatory disorders, or endometriosis, there were also significant differences between the two groups in terms of the abovementioned reproductive outcomes (Table 2). The logistic regression revealed higher odds of clinical pregnancy [crude OR: 1.20 (1.15, 1.25), P < 0.001] and live birth [crude OR: 1.26 (1.06, 1.50), P = 0.006] in the ICSI group compared with the IVF group (Table 3). Temporal trends (Fig. 2) showed marked improvements in clinical pregnancy and live birth rates from 1995 to 2018, likely reflecting advancements in ART techniques (e.g., ICSI optimization, vitrification protocols). The logistic regression analysis was then adjusted for treatment period, female age, causes of infertility, delivery history by ART treatment, number of transferred embryos, and transfer strategy. The results revealed that ICSI no longer significantly impacted on clinical pregnancy [adjusted OR: 1.01 (0.95, 1.05), P = 0.969] and live birth [adjusted OR: 1.05 (0.86, 1.28), P = 0.611]. The overall miscarriage rate was similar between the two groups (24.5% vs. 23.4%, P = 0.149), and the results remained similar when subgroup comparisons were performed. The logistic regression analysis confirmed that ICSI significantly increased the miscarriage rate with an adjusted OR of 1.15 (1.03, 1.29), P = 0.010, which was no longer the case in cycles transferring more than two embryos (Table 3).
Secondary outcomes
There were similar proportions of singletons in the ICSI and IVF groups (85.3% vs. 84.4%, P = 0.235). Unadjusted analyses revealed that ICSI was associated with reduced odds of very preterm birth, preterm birth, and post-term birth, with crude ORs of [0.65(0.43, 0.97), P = 0.037; 0.81 (0.67, 0.98), P = 0.033; and 0.87 (0.79, 0.96), P = 0.005, respectively] in singletons. After adjusting for confounders, the impact of ICSI became nonsignificant. Although ICSI significantly decreased the proportion of low weight and overweight individuals [OR: 0.52 (0.32, 0.84), P = 0.008; 0.85 (0.73, 0.98), P = 0.021], the confounders significantly attenuated the effect of ICSI on normal weight [adjusted OR: 0.90 (0.77, 1.04), P = 0.144]. Finally, ICSI had a nonsignificant effect on the sex ratio at birth with an adjusted OR of [0.91 (0.83, 1.01), P = 0.052] (Table 4). For multiple live births, ICSI was associated with a greater proportion of low birthweight, with a crude OR of [1.28 (1.01, 1.51), P = 0.003]. After adjustment for confounders, ICSI no longer impacted the birthweight. Furthermore, there were no significant differences in terms of gestational age or sex ratio at birth between the ICSI and IVF groups (Table 4).
Discussion
This retrospective study analyzed data from the HFEA registry spanning 1995–2018, focusing on FET cycles involving non-male factor infertility. After adjusting for confounders, ICSI demonstrated no significant advantages over IVF in clinical pregnancy rates (29.6% vs. 26.0%) or live birth rates (21.5% vs. 19.1%). Neonatal outcomes, including birthweight and gestational age, showed no statistically significant intergroup differences.
This retrospective study provides solid evidence on the impact of ICSI compared with IVF on the reproductive outcomes of infertile couples with non-male factor infertility, which is consistent with the evidence from studies that included patients mostly undergoing fresh embryo transfer. Both Tannus et al. and Farhi et al. investigated the impact of ICSI on reproductive outcomes in women of advanced maternal age with non-male factor infertility18,19. However, their conclusions contradict each other, which could be explained by the study design and definition of advanced age. Another two retrospective studies including patients with poor oocyte yields20,21, together with the RCT performed by Bhattacharya et al., which was the first high-quality study challenging the use of ICSI in couples with non-male factor infertility16, all of which demonstrated that ICSI did not improve reproductive outcomes in the absence of male factor infertility. However, it should be noted that the abovementioned studies all included patients with fresh embryo transfer treatment, the conditions of which could differ from those of FET treatment. For this reason, more evidence on the impact of ICSI on reproductive outcomes in couples with non-male factor infertility is warranted.
Four population-based studies recording the details of ART treatments in local places, the data of which were collected by local authorities, shed light upon the effectiveness of ICSI on reproductive outcomes in the absence of male factor infertility7,15,22,23. The studies performed by Schwarze et al. and Supramaniam et al. included cycles with fresh embryo transfer. Although both studies reported no benefits of ICSI for couples with non-male factor infertility, conflicting data exist between the two studies. While data from the Latin American Registry revealed a significantly lower live birth rate in the ICSI group than in the IVF group (22.99% vs. 28.76%)22, HFEA data paradoxically indicated increased clinical pregnancy rates (31.4% vs. 37.3%) and live birth rates (27.2% vs. 32.5%) in the ICSI group23. However, the other two population-based studies, which were able to link subsequent fresh and frozen embryo transfers to the initial retrieval cycle, made the cumulative pregnancy rate or cumulative live birth rate calculable7,15. It should be noted that although both studies demonstrated that the cumulative live birth rate of ICSI was similar to that of IVF, the reported cumulative live birth rates were mixed by fresh and frozen-thawed embryo transfer, and subgroup analyses based on fresh and frozen-thawed embryo transfers were not carried out. The freezing and thawing process can stress the embryos’ cellular structures (24). Specifically, embryos created via ICSI may be more vulnerable due to their unique developmental origins. The injection of sperm directly into the egg during ICSI could impact the embryo’s membrane integrity or cytoskeletal structure, making them potentially more sensitive to the stresses of freezing and thawing. Conventionally fertilized embryos, on the other hand, undergo natural sperm-egg interaction, which might better preserve their ability to handle cryopreservation. Their cellular machinery may be better equipped to cope with the freezing process. As a consequence, it was necessary to investigate the potential impact of insemination methods on women undergoing the FET treatment only.
Two other well-designed RCTs also investigated the impact of ICSI compared with that of IVF on the reproductive outcomes of couples with non-male factor infertility. There may be little difference with regard to early embryo development potential; however, the clinical and neonatal outcomes between the ICSI and IVF groups were similar in both studies, which led to the conclusion that the routine use of ICSI in the absence of male factor infertility was not suggested25,26. Nevertheless, the comparisons of reproductive outcomes between the ICSI and IVF groups were mixed by fresh and frozen-thawed embryo transfer in both RCTs.
Although our data were extracted from the same dataset used in the study by Supramaniam et al.23 and both studies included cycles with non-male factor infertility, the present study reported overall clinical pregnancy rates of 29.6% and 26.0% for embryos inseminated by ICSI and IVF, respectively, and overall live birth rates of 21.5% and 19.1% for embryos inseminated by and IVF, respectively, which were significantly lower than the overall clinical pregnancy rate and live birth rate reported by Supramaniam et al. These discrepancies may be explained by the fact that the freezing method was changed from slow freezing to vitrification, which is associated with better embryo development potential after the freeze‒thaw procedure, and that the ICSI procedure has been promoted since its application in clinical use, which is associated with a decreased oocyte degeneration rate. Furthermore, the study by Supramaniam et al. did not compare neonatal outcomes between the ICSI and IVF groups. These are also other important indices for assessing the efficacy and safety of insemination methods. In fact, few retrospective studies, with the exception of the study by Schwarze et al., reported neonatal outcomes22. The results revealed that ICSI was associated with significantly greater birthweight in the ICSI group when singletons were born [3064.6 (512.8) vs. 2993 (479.5), difference 71.58 (44.15 to 99.01)]. In the present study, although neonatal outcome data were used as categorical variables, comparisons between the ICSI and IVF groups were possible. Compared with IVF, ICSI was not associated with higher birthweight or full-term birth after adjustment for confounders, regardless of whether singleton or multiple live births were delivered. The inconsistent results regarding birthweight could be explained by several factors. First, the choice of ICSI as the insemination method varied dramatically between the two studies, which could be explained by the opposite proportion of cycles that were inseminated by ICSI. Under such conditions, there may be significantly different characteristics that could affect neonatal outcomes. Second, the present study included patients with FET treatment, whereas the study by Schwarze et al. included patients with fresh embryo transfer. It is well known that neonatal outcomes differ between fresh and frozen-thawed embryo transfer, which might mask the impact of ICSI on neonatal outcomes12,14. Finally, the present study included cycles spanning two decades. The large sample size made the statistical analysis more powerful.
Several population-based studies from China have demonstrated that ICSI is associated with a lower rate of male live births27,28,29,30,31. The present study confirmed that compared with IVF, ICSI is associated with a lower proportion of male live births. However, after adjusting for confounders, ICSI no longer showed significant impact on sex ratio at birth. However, the present study and the study by Supramaniam et al. included different study populations from UK reproductive centers. These two studies yielded a similar conclusion: the number of male offspring was greater in the IVF groups than in the ICSI groups during FET cycles. The possible explanation may be that embryologists select sperm based on morphology and motility, which may inadvertently favor X-bearing sperm, especially in cases of male infertility where Y sperm might be more fragile or have structural abnormalities. However, in the present study, the determined impact of ICSI on sex ratio was weakened after controlling for the confounders. On one hand, the study included couples with non-male factor infertility, which leads to embryologist’s equal selection of X- and Y-bearing sperm, in turn leading to a sex ratio closer to natural or conventional IVF levels. On the other hand, we could not divide the cycles into subgroups based on the stages of transferred embryo, which were reported to significantly impact the sex ratio.
There were several limitations of the present study. First, these retrospective HFEA data were anonymized, making it impossible to analyze the cumulative clinical pregnancy rate and cumulative live birth rate per woman being treated with FET. Nevertheless, the large sample size made the comparison of interest more powerful. Second, detailed information about the transferred embryos, such as quality and developmental stage, was not recorded, both of which would surely impact reproductive outcomes, and subgroup analyses based on the embryos were not possible. Furthermore, confounders such BMI, smoking status, and endometrial thickness were not recorded. However, considering the large sample size, the abovementioned confounders and their impacts were randomly distributed between the two groups. Third, most of the data of interest were reported as categorical variables, which limits comparisons with other studies.
In conclusion, in non-male factor FET cycles, ICSI confers no clinical advantage over IVF in reproductive or neonatal outcomes. These results underscore the need for judicious ICSI application, reserving its use for clear male factor indications.
Data availability
The data used during the current study are freely obtained from the the Human Fertilisation and Embryology Authority, and the datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Baldini, D. et al. Sperm selection for ICSI: do we have a winner? Cells. 10 (2021).
PracticeCommitteesoftheAmericanSocietyforReproductiveMedicineandtheSocietyforAssistedReproductiveTechnology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: a committee opinion. Fertil. Steril. 114, 239–245 (2020).
Palermo, G., Joris, H., Devroey, P. & Van Steirteghem, A. C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17–18 (1992).
De Geyter, C. et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum. Reprod. Open. 2020, z38 (2020).
Boulet, S. L. et al. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 313, 255 (2015).
Kim, J. Y. et al. Can intracytoplasmic sperm injection prevent total fertilization failure and enhance embryo quality in patients with non-male factor infertility? EUR. J. Obstet. Gyn R B. 178, 188–191 (2014).
Li, Z. et al. ICSI does not increase the cumulative live birth rate in non-male factor infertility. Hum. Reprod. 33, 1322–1330 (2018).
Lo, H., Weng, S. F. & Tsai, E. M. Neurodevelopmental disorders in offspring conceived via in vitro fertilization vs intracytoplasmic sperm injection. JAMA Netw. Open. 5, e2248141 (2022).
Singh, B., Reschke, L., Segars, J. & Baker, V. L. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil. Steril. 113, 252–257 (2020).
Levi-Setti, P. E., Busnelli, A., De Luca, R. & Scaravelli, G. Do strategies favoring frozen-thawed embryo transfer have an impact on differences in IVF success rate, multiple pregnancy rate, and cost per live birth between fertility clinics?? Reprod. Sci. 29, 1379–1386 (2022).
Imbar, T. et al. Reproductive outcome of fresh or frozen-thawed embryo transfer is similar in high-risk patients for ovarian hyperstimulation syndrome using GnRH agonist for final oocyte maturation and intensive luteal support. Hum. Reprod. 27, 753–759 (2012).
Wei, D. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 393, 1310–1318 (2019).
Maheshwari, A. et al. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update. 24, 35–58 (2018).
Roque, M., Haahr, T., Geber, S., Esteves, S. C. & Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update. 25, 2–14 (2019).
Iwamoto, A., Van Voorhis, B. J., Summers, K. M., Sparks, A. & Mancuso, A. C. Intracytoplasmic sperm injection vs. conventional in vitro fertilization in patients with non-male factor infertility. Fertil. Steril. 118, 465–472 (2022).
Bhattacharya, S. et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet 357, 2075–2079 (2001).
Sunderam, S., Boulet, S. L., Kawwass, J. F. & Kissin, D. M. Comparing fertilization rates from intracytoplasmic sperm injection to conventional in vitro fertilization among women of advanced age with non-male factor infertility: a meta-analysis. Fertil. Steril. 113, 354–363 (2020).
Farhi, J. et al. Should ICSI be implemented during IVF to all advanced-age patients with non-male factor subfertility? Reprod. Biol. Endocrin ;17. (2019).
Tannus, S. et al. The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum. Reprod. (2016).
Isikoglu, M. et al. Comparison of ICSI and conventional IVF in non-male factor patients with less than four oocytes. Arch. Gynecol. Obstet. 306, 493–499 (2022).
Liu, H. et al. Conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI): which is preferred for advanced age patients with five or fewer oocytes retrieved? Arch. Gynecol. Obstet. 297, 1301–1306 (2018).
Schwarze, J. et al. Is there a reason to perform ICSI in the absence of male factor? Lessons from the Latin American registry of ART. Hum. Reprod. Open. 2017 (2017).
Supramaniam, P. R. et al. ICSI does not improve reproductive outcomes in autologous ovarian response cycles with non-male factor subfertility. Hum. Reprod. 35, 583–594 (2020).
Zaat, T. et al. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst. Rev. 2, D11184 (2021).
Dang, V. Q. et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation in couples with infertility in whom the male partner has normal total sperm count and motility: an open-label, randomised controlled trial. Lancet 397, 1554–1563 (2021).
Wang, Y. et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation for couples with infertility with non-severe male factor: a multicentre, open-label, randomised controlled trial. Lancet 403, 924–934 (2024).
Wang, M. et al. Associated factors of secondary sex ratio of offspring in assisted reproductive technology: a cross-sectional study in Jilin Province, China. BMC Pregnancy Childbirth. 20, 666 (2020).
Du, T. et al. Factors affecting male-to-female ratio at birth in frozen-thawed embryo transfer cycles: a large retrospective cohort study. Front. Endocrinol. (Lausanne). 14, 1188433 (2023).
Cai, H., Ren, W., Wang, H. & Shi, J. Sex ratio imbalance following blastocyst transfer is associated with ICSI but not with IVF: an analysis of 14,892 single embryo transfer cycles. J. Assist. Reprod. Genet. 39, 211–218 (2022).
Chen, M. et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci. Rep. 7, 7754 (2017).
Bu, Z. et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China–an analysis of 121,247 babies from 18 centers. PLoS ONE. 9, e113522 (2014).
Supramaniam, P. R. et al. Secondary sex ratio in assisted reproduction: an analysis of 1 376 454 treatment cycles performed in the UK. Hum. Reprod. Open. 2019 (2019).
Acknowledgements
We thank the Human Fertilisation and Embryology Authority for providing access to and validating the data.
Funding
This work was supported to Yichun Guan by National Key R&D Program “Fertility Health and Health Security for Women and Children”: Clinical Cohort and Intervention Study on Genetic Problems in Assisted Reproduction Offspring (Grant NO: 2021YFC2700602), Yuchao Zhang and Qi Jia by Joint Construction Project of Henan Medical Science and Technology Research Plan (Grant no. LHGJ20190400 and LHGJ20210467).
Author information
Authors and Affiliations
Contributions
YC.Z. and YL. L. wrote the main manuscript text and YC.Z. and Q. J. prepared Figs. 1 and 2; Tables 1, 2, 3 and 4. YC.G. designed and approved the study. All authors reviewed the manuscrip.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Jia, Q., Liu, Y. et al. Insemination methods for embryos transferred in frozen-thawed embryo transfer cycles do not impact reproductive outcomes in couples with non-male factor infertility. Sci Rep 15, 13630 (2025). https://doi.org/10.1038/s41598-025-97051-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97051-x