Abstract
Background
Natural cycle in vitro fertilization (NC-IVF) represents a convenient and safe assisted reproductive technology, making it particularly advantageous for patients with poor ovarian response (POR). This research evaluates the effectiveness of NC-IVF for women with POR, aiming to inform personalized treatment decisions.
Method
This retrospective cohort study encompassed 13,013 cycles involving women diagnosed with poor ovarian response according to the Bologna criteria. These patients underwent either natural cycles or controlled ovarian stimulation cycles. The primary outcome measure was the cumulative live birth rates, and the secondary outcomes included laboratory and clinical outcomes.
Results
A total of 1073 natural cycles and 11,940 COS cycles were analyzed, with 5956 undergoing low-dose gonadotropin treatment and 5984 receiving high-dose gonadotropin. The basic characteristics were comparable among the three groups. In both fresh and frozen embryo transfer cycles, clinical pregnancy rates, implantation rates, and live birth rates were comparable across all three groups. Furthermore, no statistically significant differences were observed in cumulative live birth rates or time to first live birth between the groups examined. Expenditures in the natural cycle group were substantially lower than those in both COS cohorts. Importantly, further analysis indicated that there were no significant differences among the three groups concerning either pregnancy complications or neonatal outcomes.
Conclusion
Our findings indicate that for women demonstrating a poor ovarian response, NC treatment yields comparable pregnancy and live birth rates when compared to controlled ovarian stimulation methods. The natural cycle represents a safe, effective, and economically viable treatment option for this patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Infertility affects a significant proportion of the reproductive-age population worldwide. Assisted reproductive technology (ART), including in vitro fertilization (IVF), has fundamentally transformed the landscape of infertility treatment [1]. Despite substantial advancements in ART since its inception in 1978, several issues remain unresolved. Notably, the clinical management of patients exhibiting poor ovarian response (POR) continues to present challenges in everyday practice, leading to frustration for both patients and fertility specialists. This is characterized by inadequate ovarian stimulation and compromised oocyte retrieval during conventional IVF cycles [2]. The reported incidence of POR ranges from approximately 9 to 24% [3], resulting in high rates of cycle cancellations and reduced live birth rates—both factors that significantly contribute to IVF failure. Controlled ovarian stimulation (COS) protocols aim to increase the number of oocytes through exogenous gonadotropin administration; however, they often encounter difficulties when applied to patients with POR. Consequently, this frequently leads to cycle cancellations or suboptimal outcomes. Such setbacks can impose considerable emotional distress, financial burdens, and potential iatrogenic risks on affected individuals [4].
Current management strategies for patients with poor ovarian response (POR) primarily emphasize the optimization of competent oocytes or embryos. However, the inherent limitations of controlled ovarian stimulation (COS) protocols within this patient population necessitate the exploration of alternative approaches that are gentler, more physiological, and tailored to individual needs. Natural cycle in vitro fertilization (NC-IVF) presents a compelling alternative [5, 6]. NC-IVF is characterized by its reliance on the patient’s intrinsic ovarian function, thereby eliminating the need for exogenous hormonal stimulation [7]. This methodology removes potentially stressful and invasive injections associated with COS, which enhances patient compliance and reduces psychological distress [8]. This consideration is particularly pertinent for women with POR, who face an increased risk of adverse events during ovarian stimulation. Furthermore, emerging evidence suggests that oocytes retrieved during natural cycles may demonstrate superior developmental competence compared to those obtained following controlled ovarian stimulation [9]. The potential for enhanced fertilization and embryo development—coupled with reduced costs associated with this less complex protocol—positions NC-IVF as a more economically viable option for both patients and healthcare services [10].
The etiopathogenesis of poor ovarian response (POR) is intricate and only partially understood. Nevertheless, several recognized etiological factors include age-related depletion of ovarian follicles, advanced endometriosis, chromosomal and genetic alterations, previous ovarian surgeries and pelvic adhesions, metabolic and enzymatic disorders, as well as toxic, autoimmune, and infectious diseases. Several genes involved in ovarian development and function, with variants reported to contribute to poor ovarian response, participate in key biological processes including gonadal development, meiosis, folliculogenesis, and ovulation [11]. In recent decades, numerous studies have explored various approaches for the management of POR. It has been hypothesized in some studies that pretreatment with growth hormone or androgens may enhance ovarian responsiveness and embryo quality [12, 13]; however, they have not succeeded in identifying unequivocally effective strategies. The absence of conclusive evidence primarily stems from significant discrepancies in the definitions of PORs. This variation complicates the comparison of studies and their findings considerably. Several retrospective studies have indicated that, in patients with poor ovarian response (POR), natural cycle IVF (NC-IVF) demonstrates comparable implantation and pregnancy rates to the conventional controlled ovarian stimulation (COS) protocol [14, 15]. However, current research presents conflicting results regarding live birth rates following NC-IVF. Some studies report significantly lower live birth rates with NC-IVF compared to the COS protocol, while others find no significant difference [8, 16]. The number of prospective randomized controlled trials (RCTs) investigating the use of NC-IVF in POR patients is limited. Our study aims to explore the effectiveness of NC-IVF in this patient population through a large-scale investigation.
This retrospective cohort study was conducted to evaluate the impact of natural cycle IVF on laboratory and pregnancy outcomes among women diagnosed with POR. Our primary objective is to refine the clinical application of NC-IVF and inform treatment strategies for women experiencing severe POR, thereby optimizing their prospects for successful reproductive outcomes.
Materials and methods
This retrospective cohort study enrolled 11,346 women with POR who underwent 13,013 cycles between 2015 and 2023 at the Center for Reproductive Medicine, Peking University Third Hospital. The study received ethical approval from the institutional review board at Peking University Third Hospital (No. IRB00006761-M2018002). All participants provided informed consent for the procedures involved, and all information was handled with strict confidentiality.
Study design
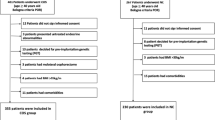
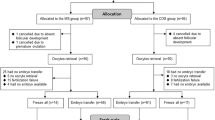
Among the 13,013 cycles (natural cycles: 1073; low-dose gonadotropin-controlled ovarian stimulation cycles: 5956; high-dose gonadotropin-controlled ovarian stimulation cycles: 5984), a total of 3774 fresh embryo transfers (FET) were performed, while 2101 cycles underwent frozen embryo transfer (Fig. 1).
Infertile women were diagnosed with poor ovarian response based on the Bologna criteria. Eligibility for inclusion required meeting at least two of the following conditions: (1) maternal age of 40 years or older or the presence of other known risk factors for poor ovarian response, (2) retrieval of three or fewer oocytes after conventional stimulation, and (3) an antral follicle count (AFC) of fewer than five to seven follicles. The AFC was determined by counting follicles measuring between 2 and 10 mm following Broekmans et al. in their study from 2010 [17]. Trained observers ensured consistent measurement of AFC through standardized workshops and procedural instructions. Patients with uterine adhesions and Müllerian anomalies (including bicornuate or complete septate uteri) were excluded from this study. Additionally, those undergoing fertility preservation and preimplantation genetic testing (PGT) were also excluded. All patients diagnosed with poor ovarian response received counseling regarding the lower clinical pregnancy rates associated with this condition. After discussing the potential benefits and risks related to natural cycle treatment versus controlled ovarian stimulation (COS), patients actively participated in selecting their preferred treatment approach.
Controlled ovarian stimulation cycle and natural cycle
Controlled ovarian stimulation was performed using a flexible GnRH antagonist protocol. FSH (Gonal-F; Serono, Aubonne, Switzerland) was initiated at a dose between 75 and 150 IU on cycle days 2–3 in low-dose COS cycles, while a higher dosage of FSH (225–300) was used in high-dose COS cycles. Adjustments to the FSH dosage were made according to the individual ovarian response, which was monitored via transvaginal ultrasonography and hormone assays including luteinizing hormone, estradiol, and progesterone. The administration of a GnRH antagonist (Cetrotide; Serono, Aubonne, Switzerland), at a subcutaneous daily dose of 0.25 mg, commenced when a follicle of at least 12 mm was identified and continued until the day of ovulation induction. For final ovulation triggering, human chorionic gonadotropin (hCG) 250 µg (Ovidrel; Serono, Aubonne, Switzerland) or triptorelin 0.2 mg (Diphereline; Ipsen, Beaufour, France) was selectively administered when 1 to 2 follicles reached a diameter of 17 mm and estradiol levels ≥ 200 pg/mL per follicle.
Natural cycles did not involve the administration of gonadotropins, clomiphene, or letrozole. The mean follicular diameter was at ≥ 17 mm, and estradiol levels were ≥ 200 pg/mL per follicle; hCG 250 µg was administered as trigger.
All patients were informed before treatment that poor ovarian response may reduce the success rate of assisted reproduction, and the clinical pregnancy rate reported in current research is less than 20% [18]. Clinician and patients engaged in shared decision-making, thoroughly discussing the details of natural cycle and COS cycles, leading to informed patient choices.
Oocyte retrieval laboratory procedures
Oocyte retrieval was performed under ultrasound guidance 34–38 h post-trigger. Transvaginal ultrasound-guided oocyte retrieval was performed using a single-lumen 17G aspiration needle under 120–130 mmHg suction pressure and intravenous sedation. Fertilization was achieved using either IVF or ICSI, as appropriate. In vitro maturation (IVM) was not performed in the study.
Embryo morphology assessment and embryo transfer
During fresh embryo transfer (ET) cycles, one or two cleavage-stage embryos, or a single blastocyst, were selected for transfer based on the professional judgment of the attending physicians and embryologists. Surplus embryos were subsequently cryopreserved for potential future use. All embryos were cultured under controlled conditions at 37 °C in an atmosphere of 5% O2 and 6% CO2. Embryo development was assessed based on established morphological criteria. Specifically, cleavage-stage embryos (day 2 or 3 with ≥ 3 or ≥ 6 blastomeres, respectively, and < 20% fragmentation) and blastocysts (ranging from fully expanded to hatched with inner cell mass and trophectoderm quality ≥ 4BC) were deemed suitable for transfer or cryopreservation.
Frozen embryo transfer
A standardized endometrial preparation protocol was utilized in all frozen embryo transfer (FET) cycles. Endometrial priming consisted of a natural cycle, a stimulated cycle, and a hormone replacement cycle therapy. Embryo transfer, involving one or two cleavage-stage embryos or a single blastocyst, occurred between days 5 and 7 following progesterone commencement.
Luteal phase support
Luteal phase support varied based on the embryo transfer cycle type. For luteal phase support in fresh ET cycles, vaginal progesterone gel (Crinone, Merck Serono, Switzerland) 90 mg/day was administered vaginally, starting on the day of oocyte retrieval until 10–12 weeks of gestation if pregnancy was achieved. When using triptorelin for ovulation induction, luteal phase support is intensified with a regimen of vaginal progesterone, oral dydrogesterone (40 mg daily), and estradiol valerate (Progynova, Bayer, Germany, 3 mg daily). For luteal phase support in natural and stimulated FET cycles, oral dydrogesterone (Duphaston, Abbott, OLST, Netherlands, 40 mg daily) was initiated on the day of ovulation and continued until 10–12 weeks of gestation if pregnancy was achieved. In hormone replacement therapy (HRT) frozen embryo transfer (FET) cycles, once the timing of FET was established, a regimen of vaginal progesterone (Crinone gel 8%, Merck Serono SA, 90 mg daily) and oral dydrogesterone (40 mg daily) was initiated.
Variables and outcome measures
Baseline demographic and clinical characteristics of eligible participants were collected, including age, body mass index (BMI; kg/m2), duration of infertility, infertility years, and number of IVF attempts.
Laboratory and treatment measures encompassed levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and anti-Müllerian hormone (AMH) at baseline and FSH, progesterone (P), and LH levels on the hCG trigger day. Duration of the follicular phase and the total gonadotropin (Gn) dose were administered.
Fertilization outcomes, including the number of oocytes retrieved, two-pronuclear zygotes, available embryos, good quality embryos, and transferred embryos, were assessed for all participants. Comparative analyses were performed to determine differences in these endpoints among the three groups.
Pregnancy outcomes were assessed by positive β-hCG test (serum hCG ≥ 5 mIU/mL [19]), clinical pregnancy (ultrasonographic confirmation of a gestational sac), and live birth (delivery of a live infant after 22 weeks of gestation or with a birth weight > 1000 g). For successful deliveries, gestational age at delivery (preterm or term) was recorded. Safety outcomes included miscarriage, ectopic pregnancy, and obstetric and perinatal complications.
Statistical analysis
Continuous variables were presented as means with standard deviations (SD) when normally distributed and compared using Student’s t-tests. Continuous variables that did not follow a normal distribution were summarized using medians with interquartile ranges (IQR), and group comparisons were performed using Mann–Whitney U tests. Categorical data were presented as proportions and analyzed for intergroup differences using either Pearson’s chi-squared test or Fisher’s exact test, as dictated by sample size and distribution. All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA), and statistical significance was set at p < 0.05 with a two-sided test.
Results
Participants
In total, 13,013 cycles were included, comprising 1073 natural cycles, 5956 LDOS cycles, and 5984 HDOS cycles. The number of natural cycles, LDOS, and HDOS cycles performed each year is shown in Table 1. The proportion of natural cycles remained stable before 2020 but has shown an increasing trend since then.
The demographic and clinical characteristics of the study population
As shown in Table 2, the mean age of the women was 38.58 ± 5.65 years in the natural cycle group, while 38.61 ± 5.14 years in the LDOS group and 38.32 ± 5.23 years in the HDOS group. Other baseline profiles, such as body mass index (BMI), duration and type of infertility, antral follicle count (AFC), and basal follicle-stimulating hormone (FSH) concentrations, were comparable among the three groups. However, women in the natural cycle group exhibited significantly higher baseline LH levels (median: 4.10 mIU/mL; IQR: 2.45–7.15) and lower baseline AMH levels (median: 0.26 ng/mL; IQR: 0.11–0.62).
Treatment and laboratory outcomes
As presented in Table 3, the treatment and laboratory outcomes are detailed. As anticipated, patients undergoing natural cycles experienced a median of 14 days in the follicular phase without gonadotropin stimulation. In contrast, patients receiving low-dose ovarian stimulation (LDOS) and high-dose ovarian stimulation (HDOS) were administered a median of 1625 IU and 3600 IU of gonadotropins, respectively. The median number of days using gonadotropins was 11.0 and 12.0 days in the LDOS and HDOS groups, respectively. Consequently, levels of estradiol and progesterone were significantly elevated, while levels of luteinizing hormone were significantly lower, during controlled ovarian stimulation (COS) cycles compared to natural cycles. Cycle cancellation rate in the NC group (26.8%) was significantly higher than in the LDOS (8.0%) and HDOS (7.6%) groups (p < 0.01). The proportions of individuals over 40 years old in the NC, LDOS, and HDOS groups were 58.7%, 53.8%, and 46.6%, respectively, with the differences statistically significant (p = 0.004). The primary reasons for cycle cancellation included premature ovulation, absent follicle growth, patient preference, and failure to retrieve oocytes. Detailed information is presented in Supplementary Table 1.
Significant differences (p < 0.01) were observed between natural cycles and COS cycles regarding the number of oocytes retrieved, fertilized oocytes at the two-pronuclear stage (2PN), available embryos, and good quality embryos. The COS cycles resulted in a greater quantity of both oocytes retrieved and available embryos while demonstrating higher rates for oocyte retrieval and embryo transfer. Notably, both LDOS and HDOS groups yielded comparable numbers concerning oocytes retrieved as well as embryos available for transfer. A significantly higher ratio of good quality embryos was observed in the NC group (89.5%) compared to the LDOS (84.5%) and HDOS (82.6%) groups (p < 0.01). It is important to highlight that the fresh embryo transfer rate was significantly higher in natural cycles (68.5%) when compared to both LDOS (58.1%) and HDOS (51.9%) groups (p < 0.01).
Pregnancy outcomes
The pregnancy outcomes of fresh embryo transfer (ET) cycles are summarized in Table 4. Among the initial 13,013 cycles, a total of 3774 fresh ET cycles were conducted. Clinical pregnancies were achieved in 40 cases (19.1%) within natural cycles, 396 cases (21.3%) in the LDOS cycles, and 384 cases (22.5%) in the HDOS cycles; however, no statistically significant differences were observed among these groups (p = 0.437). The implantation rates also exhibited similarity across the three categories: 14.8% for natural cycles, 15.1% for the LDOS cycles, and 15.4% for the HDOS cycles. Live birth rates recorded were as follows: 16.7% (35 cases) in the natural cycle group, 17.5% (326 cases) in the LDOS group, and 18.8% (320 cases) in the HDOS group—again showing no statistically significant difference (p = 0.550).
Cumulative results for complete treatment cycles that included frozen embryo transfer (FET) are presented in Table 5. A total of 3019 FET procedures were performed—comprising 71 from natural cycle groups, and respectively, 982 from the LDOS groups, and 1048 from the HDOS groups. Clinical pregnancies occurred in 18 cases (25.4%) of the natural cycle FET, compared to 26.8% in the LDOS group and 27.3% in the HDOS group; no significant difference was found (p = 0.921). Implantation rates remained comparable across all groups. Within complete treatment cycles, the live birth rates were 19.7% in the natural cycle group, 20.5% in the LDOS group (p = 1.000), and 21.1% in the HDOS group (p = 0.881), with no statistically significant differences noted.
In Table 6, a comprehensive analysis was performed on patients who achieved a live birth. This analysis indicated that the cumulative live birth rates (CLBRs) were 16.1% for the natural cycle group, 16.5% for the LDOS group, and 16.5% for the HDOS group. No statistically significant differences in cumulative live birth rates were observed among the three groups (p = 0.985). Additionally, the median time to first live birth was comparable across all groups, with an average duration of 3 years. The median medication costs were ¥192 for the natural cycle group, ¥3312 for the LDOS group, and ¥5412 for the HDOS group, revealing a statistically significant difference (p < 0.01). The medication costs associated with the natural cycle were significantly lower than those of both the LDOS and HDOS groups. Furthermore, no statistically significant differences in pregnancy complications or neonatal outcomes were noted among the three groups.
Discussion
Through this retrospective study, we found that COS significantly enhanced the oocyte retrieval rate and the rate of viable embryo development in patients with POR. Although there was an increase in LBRs in COS protocol, the financial cost associated with natural cycle treatment was lower. Despite the observed improvement in LBRs achieved with COS, the natural cycle approach presented a more economically advantageous alternative. These findings support the use of a natural cycle protocol as an economically effective and appropriate treatment option for infertile women experiencing POR.
In adherence to the treatment protocols of Peking University Third Hospital and respecting patients’ individual preferences, attending physicians engaged in thorough discussions with patients, outlining the benefits and limitations of various treatment options. Natural cycle provides temporal flexibility, allowing for sequential oocyte retrievals in subsequent cycles, thereby facilitating multiple treatment attempts. The reduced need for medication inherent in NC-IVF confers dual benefits of diminished treatment costs and mitigation of physiological and psychological side effects related to pharmacotherapy. While health insurance covered the expenses associated with oocyte retrieval, laboratory culture, and embryo transfer, the relatively high cost of ovarian stimulation drugs made the more cost-effective natural cycle a preferable option, broadening accessibility and affordability.
Among natural cycles with transferable embryos, 31.5% chose embryos cryopreservation. Due to the low oocyte yield and subsequent limited number of embryos obtained in patients with POR, patients may wish to accumulate embryos prior to transfer. Furthermore, some patients presented with hydrosalpinx, unfavorable endometrial conditions, or elevated post-ovulation induction progesterone levels that could negatively impact endometrial receptivity. In the NC group, 71 cases (74.0%) have returned for their transfers, compared to 982 cases (73.2%) in the LDOS group and 1048 cases (66.2%) in the HDOS group.
While NC-IVF exhibits lower pregnancy rates per cycle compared to conventionally stimulated cycles, this is attributable to higher cycle cancellation rates and fewer oocytes retrieved. However, per-embryo pregnancy rates in natural cycles are not significantly different from those in COS cycles. Once embryos are obtained in natural cycles, they frequently demonstrate higher quality and are associated with comparable pregnancy and live birth rates. Gn usage in conventional stimulation protocols may potentially compromise embryo quality and developmental competence by impacting cleavage ability [20]. Research by Lu et al. [21] suggests that the absence of exogenous hormonal stimulation in NC-IVF cycles, allowing oocyte maturation under physiological hormonal influences, does not lead to significantly elevated aneuploidy rates. Instead, the resulting embryos often demonstrate superior quality compared to conventional IVF cycles. The lack of pharmaceutical intervention in natural cycles minimizes potential impact on follicle development, potentially leading to embryos with superior developmental potential. Ultimately, the pregnancy success rates in natural cycles are effectively assured only when viable embryos are obtained.
In natural cycles, rising estrogen levels trigger a sharp LH surge, which is critical for final oocyte maturation and ovulation. This LH surge is characterized by a brief duration but high amplitude, playing a significant role in luteinizing the dominant follicle [22]. In contrast, COS cycles typically bypass the natural LH surge, instead relying on hCG administration to trigger final oocyte maturation and ovulation. While a natural cycle usually involves the development and ovulation of a single dominant follicle whose granulosa cells luteinize to form the corpus luteum and secrete progesterone, COS cycles stimulate the synchronous development of multiple follicles [23]. Although the LH stimulus per follicle in COS may be less intense than the LH surge in a natural cycle, the sheer number of follicles undergoing luteinization results in a significantly greater overall mass of luteinized granulosa cells, leading to higher progesterone levels in COS cycles.
The primary reasons for cancellation were premature ovulation, absent follicle growth, and patient preference. Premature LH surges, leading to premature follicular rupture and increased cycle cancellation rates, are a major cause of failure in NC-IVF. Determining the appropriate oocyte retrieval timing under varying LH levels in natural cycles is crucial for improving success rates, necessitating close hormonal monitoring and timely oocyte retrieval when the follicle size is optimal. Combining natural cycle IVF with in vitro maturation (IVM) of immature oocytes can effectively compensate for the low oocyte yield. Bartolacci et al. reported that the application of IVM to metaphase I oocytes has the potential to augment the quantity of utilizable oocytes and embryos in POR patients, subsequently enhancing the overall efficacy of the therapeutic process [24].
For patients with POR, natural intercourse is virtually the only option without utilizing ART, resulting in extremely low pregnancy success rates. NC-IVF, which can be performed continuously each month with minimal side effects [25], imposes a low physical and psychological burden on patients and is a cost-effective treatment. Moreover, a large portion of the cost is often covered by insurance. For patients with severe conditions and financial constraints, NC-IVF provides an accessible and effective treatment option. Haemorli Keller et al. [26] demonstrated that patients undergoing NC-IVF experienced significantly lower levels of depression and higher treatment satisfaction compared to those undergoing conventional IVF.
AMH is independent of the menstrual cycle and widely used as a marker of ovarian reserve. Although serum AMH concentrations decline with advancing age, studies indicate its limited predictive capacity for outcomes following natural cycle IVF [27]. A 2013 study also reported no significant differences in NC-IVF cycle outcomes—specifically cycle cancellation, oocyte retrieval, biochemical and clinical pregnancy, and live birth—when comparing patients with AMH levels ≤ 0.5 ng/mL to those with AMH > 2.04 ng/mL [28].
Early animal studies suggested that high estrogen levels during superovulation may induce oocyte DNA damage and aneuploidy, compromise artery remodeling, and culminate in placental dysplasia, thereby elevating the risk of adverse obstetric outcomes [29, 30]. NC-IVF’s closer-to-physiological estrogen and progesterone levels may lead to better obstetric outcomes compared to COS cycles. Studies have demonstrated that non-embryonic ploidy rates are comparable between natural cycle and COS cycle [31], while a 1.27-fold higher incidence of preterm birth and a 1.95-fold higher incidence of low-birth-weight infants with COS cycles compared to natural cycles were demonstrated [32]. Although no statistically significant differences in preterm birth or low birth weight were observed between groups, the possibility that this finding is attributable to the study’s limited sample size cannot be excluded.
This study is strengthened by its considerable sample size and its specific focus on older women meeting the Bologna criteria for POR. Clinicians and these patients alike are often confronted with the prospect of repeated stimulation cycles before initiating IVF treatment. Indeed, the COS group comprises women who have undergone multiple treatment cycles. A further strength lies in our evaluation of CLBR in modified natural cycles. In line with the suggestion by Datta et al. the accumulation of embryos over three NC-IVF cycles, prior to transfer, may mitigate the risk of transfer failure and improve subsequent clinical pregnancy and live birth rates [33]. Therefore, this analysis provides valuable data for informing clinical decisions regarding ovarian stimulation strategies for POR patients.
The retrospective methodology of this study is a notable limitation, as it is susceptible to selection bias and the effects of unaccounted for confounding factors. Treatment assignment was based on physician judgment and patient preference, rather than randomization. Meanwhile, it is believed that lifestyle and environmental factors, such as smoking and obesity, can adversely affect fertility [34]. In our research, we evaluated the BMI of the study population but did not include smoking, which will be considered in the future study. Precise determination of the optimal timing for oocyte retrieval in natural cycles, coupled with a rational selection of trigger agents, is crucial for maximizing oocyte yields. The integration of IVM may subsequently improve the pregnancy rates of oocytes retrieved from small follicles. Further research into the impact of the ovarian microenvironment, metabolic parameters, and inflammatory mediators on NC-IVF outcomes among patients with POR is warranted.
In conclusion, our study demonstrates that natural cycle IVF offers comparable pregnancy and live birth rates to controlled ovarian stimulation in women with a poor ovarian response. Given the potential for complications associated with excessive stimulation, natural cycle IVF may represent a more judicious approach for select patients. As the clinical application of natural cycle IVF expands, and the evidence base for its safety and efficacy strengthens, continued research is imperative to refine techniques and optimize outcomes for this unique patient population.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Herbert M, Choudhary M, Zander-Fox D. Assisted reproductive technologies at the nexus of fertility treatment and disease prevention. Science. 2023;380(6641):164–7.
La Marca A, Grisendi V, Giulini S, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet. 2015;32(6):931–7.
Vaiarelli A, Cimadomo D, Ubaldi N, et al. What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol. 2018;30(3):155–62.
Mizrachi Y, Horowitz E, Farhi J, et al. Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update. 2020;26(1):118–35.
Zhang Y, Zhang C, Shu J, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. 2020;26(2):247–63.
Lee YX, Shen MS, Tzeng CR. Low dose growth hormone adjuvant treatment with ultra-long ovarian stimulation protocol in poor responders showed non-inferior pregnancy outcome compared with normal responders. Front Endocrinol (Lausanne). 2019;10:892.
Practice Committee of the American Society for Reproductive Medicine. Comparison of pregnancy rates for poor responders using IVF with mild ovarian stimulation versus conventional IVF: a guideline. Fertil Steril. 2018;109(6): 993–9.
Polyzos NP, Blockeel C, Verpoest W, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012;27(12):3481–6.
Kadoch IJ, Phillips SJ, Bissonnette F. Modified natural-cycle in vitro fertilization should be considered as the first approach in young poor responders. Fertil Steril. 2011;96(5):1066–8.
Kedem A, Tsur A, Haas J, et al. Is the modified natural in vitro fertilization cycle justified in patients with “genuine” poor response to controlled ovarian hyperstimulation? Fertil Steril. 2014;101(6):1624–8.
Ke H, Tang S, Guo T, et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat Med. 2023;29(2):483–92.
de Ziegler D, Streuli I, Meldrum DR, et al. The value of growth hormone supplements in ART for poor ovarian responders. Fertil Steril. 2011;96(5):1069–76.
Yeung TW, Chai J, Li RH, et al. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil Steril. 2014;102(1):108-115.e1.
Elizur SE, Aslan D, Shulman A, et al. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J Assist Reprod Genet. 2005;22(2):75–9.
Ata B, Yakin K, Balaban B, et al. Embryo implantation rates in natural and stimulated assisted reproduction treatment cycles in poor responders. Reprod Biomed Online. 2008;17(2):207–12.
Liu Y, Su R, Wu Y. Cumulative live birth rate and cost-effectiveness analysis of gonadotropin releasing hormone-antagonist protocol and multiple minimal ovarian stimulation in poor responders. Front Endocrinol (Lausanne). 2020;11: 605939.
Ezra O, Haas J, Nahum R, et al. Do poor-responder patients undergoing IVF benefit from splitting and increasing the daily gonadotropin dose? Gynecol Endocrinol. 2019;35(10):890–3.
Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
Yang R, Yang S, Li R, et al. Biochemical pregnancy and spontaneous abortion in first IVF cycles are negative predictors for subsequent cycles: an over 10,000 cases cohort study. Arch Gynecol Obstet. 2015;292(2):453–8.
Ziebe S, Bangsbøll S, Schmidt KL, et al. Embryo quality in natural versus stimulated IVF cycles. Hum Reprod. 2004;19(6):1457–60.
Lu CL, Yan ZQ, Song XL, et al. Effect of exogenous gonadotropin on the transcriptome of human granulosa cells and follicular fluid hormone profiles. Reprod Biol Endocrinol. 2019;17(1):49.
de Ziegler D, Fraisse T, de Candolle G, et al. Outlook: Roles of FSH and LH during the follicular phase: insight into natural cycle IVF. Reprod Biomed Online. 2007;15(5):507–13.
Adda-Herzog E, Poulain M, de Ziegler D, et al. Premature progesterone elevation in controlled ovarian stimulation: to make a long story short. Fertil Steril. 2018;109(4):563–70.
Bartolacci A, Busnelli A, Pagliardini L, et al. Assessing the developmental competence of oocytes matured following rescue in vitro maturation: a systematic review and meta-analysis. J Assist Reprod Genet. 2024;41(8):1939–50.
von Wolff M. The role of Natural Cycle IVF in assisted reproduction. Best Pract Res Clin Endocrinol Metab. 2019;33(1):35–45.
Haemmerli Keller K, Alder G, Loewer L, et al. Treatment-related psychological stress in different in vitro fertilization therapies with and without gonadotropin stimulation. Acta Obstet Gynecol Scand. 2018;97(3):269–76.
Ovarian Stimulation T, Bosch E, Broer S, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(†). Hum Reprod Open. 2020;2020(2):hoaa009.
von Wolff M, Hua YZ, Santi A, et al. Follicle flushing in monofollicular in vitro fertilization almost doubles the number of transferable embryos. Acta Obstet Gynecol Scand. 2013;92(3):346–8.
Roberts R, Iatropoulou A, Ciantar D, et al. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72(1):107–18.
Mainigi MA, Olalere D, Burd I, et al. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90(2):26.
Hong KH, Franasiak JM, Werner MM, et al. Embryonic aneuploidy rates are equivalent in natural cycles and gonadotropin-stimulated cycles. Fertil Steril. 2019;112(4):670–6.
Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–41.
Datta AK, Campbell S, Felix N, et al. Accumulation of embryos over 3 natural modified IVF (ICSI) cycles followed by transfer to improve the outcome of poor responders. Facts Views Vis Obgyn. 2019;11(1):77–84.
Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76.
Funding
Capital’s Funds for Health Improvement and Research of Beijing (2024-2-40911), National Natural Science Foundation of China (82201805, 82171632), National key technologies research and development program (2021YFC2700605).
Author information
Authors and Affiliations
Contributions
YR conceived and designed the study. LJ analyzed the data and drafted the manuscript. CL collected the data. GW, TT, ZX, LR, and QJ participated in the revision process and approved this submission for publication.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This retrospective study was approved by the Ethics Committee of Peking University Third Hospital (No. IRB00006761-M2020004), and the methods were carried out in accordance with the approved guidelines. All the patients have been informed and signed informing consent before the experiments.
Conflict of interest
The authors declare no competing interests.
Attestation statements
Data regarding any of the subjects in the study has not been previously published unless specified.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18.8 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, J., Guo, W., Tian, T. et al. Cumulative live birth rates among over 13,000 poor ovarian responders from 2015 to 2023: a retrospective cohort study assessing the efficacy of natural cycle and controlled ovarian stimulation. J Assist Reprod Genet (2025). https://doi.org/10.1007/s10815-025-03544-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-025-03544-z