Abstract
Purpose
Does vitrification/warming affect the mitochondrial DNA (mtDNA) content and the gene expression profile of blastocysts?
Methods
Prospective cohort study in which 89 blastocysts were obtained from 50 patients between July 2017 and August 2018. mtDNA was measured in a total of 71 aneuploid blastocysts by means of real-time polymerase chain reaction (RT-PCR). Transcriptomic analysis was performed by RNA sequencing (RNA-seq) in an additional 8 aneuploid blastocysts cultured for 0 h after warming, and 10 aneuploid blastocysts cultured for 4–5 h after warming.
Results
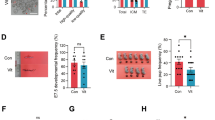
A significant decrease in mtDNA content just during the first hour after the warming process in blastocysts was found (P < 0.05). However, mtDNA content experimented a significantly increased along the later culture hours achieving the original mtDNA levels before vitrification after 4–5 h of culture (P < 0.05). Gene expression analysis and functional enrichment analysis revealed that such recovery was accompanied by upregulation of pathways associated with embryo developmental capacity and uterine embryo development. Interestingly, the significant increase in mtDNA content observed in blastocysts just after warming also coincided with the differential expression of several cellular stress response-related pathways, such as apoptosis, DNA damage, humoral immune responses, and cancer.
Conclusion
To our knowledge, this is the first study demonstrating in humans, a modulation in blastocysts mtDNA content in response to vitrification and warming. These results will be useful in understanding which pathways and mechanisms may be activated in human blastocysts following vitrification and warming before a transfer.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
Data regarding any of the subjects in the study have not been previously published.
References
Bosch E, De Vos M, Humaidan P. The future of cryopreservation in assisted reproductive technologies. Front Endocrinol (Lausanne). 2020;11:1–15.
De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2015: results generated from European registries by ESHRE†. Hum Reprod Open. 2020;2020:1–17.
Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab. 2011;22:412–20.
Meistermann D, Bruneau A, Loubersac S, Reignier A, Firmin J, François-Campion V, et al. Integrated pseudotime analysis of human pre-implantation embryo single-cell transcriptomes reveals the dynamics of lineage specification. Cell Stem Cell. 2021;28:1625-1640.e6.
Feuer S, Rinaudo P. Preimplantation stress and development. Birth Defects Res Part C - Embryo Today Rev. 2012;96:299–314.
Ramos-Ibeas P, Heras S, Gómez-Redondo I, Planells B, Fernández-González R, Pericuesta E, et al. Embryo responses to stress induced by assisted reproductive technologies. Mol Reprod Dev. 2019;86:1292–306.
Breton-Larrivée M, Elder E, McGraw S. DNA methylation, environmental exposures and early embryo development. Anim Reprod. 2019;16:465–74.
Gualtieri R, Kalthur G, Barbato V, Di Nardo M, Adiga SK, Talevi R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants. 2021;10:1–23.
Nohales-Córcoles M, Sevillano-Almerich G, Di Emidio G, Tatone C, Cobo AC, Dumollard R, et al. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum Reprod. 2016;31:1850–8.
Zhao XM, Du WH, Wang D, Hao HS, Liu Y, Qin T, et al. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol Reprod Dev. 2011;78:942–50.
Amoushahi M, Salehnia M, Mowla SJ. Vitrification of mouse MII oocyte decreases the mitochondrial DNA copy number, TFAM gene expression and mitochondrial enzyme activity. J Reprod Infertil. 2017;18:343–51.
Xu J, Zhang D, Ju S, Sun L, Zhang S, Wu C, et al. Mitophagy is involved in the mitochondrial dysfunction of vitrified porcine oocytes. Mol Reprod Dev. 2021;88:427–36.
Dorn GW, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–91.
Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85.
Picard M, McEwen BS. Psychological stress and mitochondria: A systematic review. Psychosom Med. 2018;80:141–53.
Valera-Alberni M, Canto C. Mitochondrial stress management: A dynamic journey. Cell Stress. 2018;2:253–74.
Tan Y, Yin X, Zhang S, Jiang H, Tan K, Li J, et al. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience. 2014;3:2047–217.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: Less is better. Fertil Steril. 2015;104:534–41.
de Los Santos MJ, Diez Juan A, Mifsud A, Mercader A, Meseguer M, Rubio C, Pellicer A. Variables associated with mitochondrial copy number in human blastocysts: what can we learn from trophectoderm biopsies? Fertil Steril. 2018;109(1):110–7. https://doi.org/10.1016/j.fertnstert.2017.09.022.
Ho JR, Arrach N, Rhodes-Long K, Salem W, McGinnis LK, Chung K, et al. Blastulation timing is associated with differential mitochondrial content in euploid embryos. J Assist Reprod Genet. 2018;35:711–20.
Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, et al. Embryonal mitochondrial dna: Relationship to embryo quality and transfer outcomes. J Assist Reprod Genet. 2018;35:871–7.
Lledo B, Ortiz JA, Morales R, García-Hernández E, Ten J, Bernabeu A, Llácer J, Bernabeu R. Comprehensive mitochondrial DNA analysis and IVF outcome. Hum Reprod Open. 2018;2018(4):hoy023. https://doi.org/10.1093/hropen/hoy02.
Kim J, Seli E. Mitochondria as a biomarker for IVF outcome. Reproduction. 2019;157:R235–42.
Scott RT, Sun L, Zhan Y, Marin D, Tao X, Seli E. Mitochondrial DNA content is not predictive of reproductive competence in euploid blastocysts. Reprod Biomed Online. 2020;41:183–90.
Fragouli E, Mitochondrial DNA. Assessment to determine oocyte and embryo viability. Semin Reprod Med. 2015;33:401–9.
Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. Obstet Gynecol Surv. 2016;11:28–9.
Clément A, Seegers V, Boucret L, Hotellier VF, Bouet PE, Descamps P, et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32:607–14.
Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32:952–62.
Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107:34–42.
Viotti M, Victor AR, Zouves CG, Barnes FL. Is mitochondrial DNA quantitation in blastocyst trophectoderm cells predictive of developmental competence and outcome in clinical IVF? J Assist Reprod Genet. 2017;34:1581–5.
Wells D. Mitochondrial DNA quantity as a biomarker for blastocyst implantation potential. Fertil Steril. 2017;108(5):742–7. https://doi.org/10.1016/j.fertnstert.2017.10.007.
Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, et al. Mitochondrial DNA quantification as a tool for embryo viability assessment: Retrospective analysis of data from single euploid blastocyst transfers. Hum Reprod. 2017;32:1282–92.
Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239–46.
Huber W, Von Heydebreck A, Sültmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002. p. 96–104.
Durbin BP, Rocke DM. Variance-stabilizing transformations for two-color microarrays. Bioinformatics. 2004;20:660–7.
Shapiro SS, Wilk MB. An analysis of variance test for normality (Complete Samples). Biometrika. 1965;52:591–611.
Royston JP. An extension of Shapiro and Wilk’s W test for normality to large samples. Appl Stat. 1982;31:115.
Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34.
Eisner V, Picard M, Hajnóczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol. 2018;20:755–65.
Sage JM, Knight KL. Human Rad51 promotes mitochondrial DNA synthesis under conditions of increased replication stress. Mitochondrion. 2013;13:350–6.
Vishnyakova PA, Volodina MA, Tarasova NV, Marey MV, Tsvirkun DV, Vavina OV, et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci Rep. 2016;6:1–9.
Wang D, Li Z, Liu W, Zhou J, Ma X, Tang J, et al. Differential mitochondrial DNA copy number in three mood states of bipolar disorder. BMC Psychiatry. 2018;18:1–8.
Chiaratti MR, Bressan FF, Ferreira CR, Caetano AR, Smith LC, Vercesi AE, et al. Embryo mitochondrial DNA depletion is reversed during early embryogenesis in cattle. Biol Reprod. 2010;82:76–85.
Matsushima Y, Goto YI, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc Natl Acad Sci U S A. 2010;107:18410–5.
Shokolenko IN, Wilson GL, Alexeyev MF. The “fast” and the “slow” modes of mitochondrial DNA degradation. Mitochondrial DNA Part A. 2014;27:490–8.
Trombly G, Said AM, Kudin AP, Peeva V, Altmüller J, Becker K, et al. The fate of oxidative strand breaks in mitochondrial DNA. Antioxidants. 2023;12:1087.
Xiao X, Wu ZC, Chou KC. A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PLoS One. 2011;6:e20592.
Kai Y, Takamatsu C, Tokuda K, Okamoto M, Irita K, Takahashi S. Rapid and random turnover of mitochondrial DNA in rat hepatocytes of primary culture. Mitochondrion. 2006;6:299–304.
Zhao L. Mitochondrial DNA degradation: A quality control measure for mitochondrial genome maintenance and stress response. Enzymes. 2019;45:311–41.
Thundathil J, Filion F, Smith LC. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev. 2005;71:405–13.
El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131(2):233–45. https://doi.org/10.1530/rep.1.00551.
McConnell JML, Petrie L. Mitochondrial DNA turnover occurs during preimplantation development and can be modulated by environmental factors. Reprod Biomed Online. 2004;9:418–24.
Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo JA, Munné S, et al. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: A blinded prospective non-selection study. Hum Reprod. 2017;32:2340–7.
Monnot S, Samuels DC, Hesters L, Frydman N, Gigarel N, Burlet P, et al. Mutation dependance of the mitochondrial DNA copy number in the first stages of human embryogenesis. Hum Mol Genet. 2013;22:1867–72.
Chatzimeletiou K, Sioga A, Petrogiannis N, Panagiotidis Y, Prapa M, Patrikiou A, et al. Viability assessment using fluorescent markers and ultrastructure of human biopsied embryos vitrified in open and closed systems. Reprod Biomed Online. 2021;43:833–42.
Boles NC, Hirsch SE, Le S, Corneo B, Najm F, Minotti AP, et al. NPTX1 Regulates neural lineage specification from human pluripotent stem cells. Cell Rep. 2014;6:724–36.
Li Y, Liu X, Chen Z, Song D, Yang J, Zuo X, et al. Effect of follistatin on pre-implantational development of pig parthenogenetic embryos. Anim Sci J. 2018;89:316–27.
Breckenridge RA, Kelly P, Nandi M, Vallance PJ, Mohun TJ, Leiper J. A role for Dimethylarginine Dimethylaminohydrolase 1 (DDAH1) in mammalian development. Int J Dev Biol. 2010;54:215–20.
Zeng TB, Pierce N, Liao J, Singh P, Lau K, Zhou W, et al. EHMT2 suppresses the variation of transcriptional switches in the mouse embryo. PLoS Genet. 2021;17:e1009908.
Zeng T-B, Pierce N, Liao J, Singh P, Zhou W, Szabó PE. EHMT2 controls transcriptional noise and the developmental switch after gastrulation in the mouse embryo. bioRxiv. 2021. https://doi.org/10.1101/2021.03.29.437567
Gupta A, Singh J, Dufort I, Robert C, Dias FCF, Anzar M. Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage. PLoS One. 2017;12:1–20.
Krupenko NI, Sharma J, Pediaditakis P, Helke KL, Hall MS, Du X, et al. Aldh1l2 knockout mouse metabolomics links the loss of the mitochondrial folate enzyme to deregulation of a lipid metabolism observed in rare human disorder. Hum Genomics. 2020;14:1–15.
Malkowska A, Penfold C, Bergmann S, Boroviak TE. A hexa-species transcriptome atlas of mammalian embryogenesis delineates metabolic regulation across three different implantation modes. Nat Commun. 2022;13(1):3407. https://doi.org/10.1038/s41467-022-30194-x.
Shaw L, Sneddon SF, Brison DR, Kimber SJ. Comparison of gene expression in fresh and frozen-thawed human preimplantation embryos. Reproduction. 2012;144:569–82.
Cuello C, Martinez CA, Cambra JM, Parrilla I, Rodriguez-Martinez H, Gil MA, et al. Effects of vitrification on the blastocyst gene expression profile in a porcine model. Int J Mol Sci. 2021;22:1–14.
Li J, Zhu L, Huang J, Liu W, Han W, Huang G. Long-Term storage does not Affect the expression profiles of mRNA and long non-coding RNA in vitrified-warmed human embryos. Front Genet. 2022;12:1–12.
Gurung S, Greening DW, Catt S, Salamonsen L, Evans J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol Hum Reprod. 2020;26:510–20.
Shen H, Yang M, Li S, Zhang J, Peng B, Wang C, et al. Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell. 2021;184:2843–59.
Zhu M, Shahbazi MN, Martin A, Zhang C, Sozen B, Borsos M, et al. Human embryo polarization requires plc signaling to mediate trophectoderm specification. Elife. 2021;10:e65068.
Derakhshan-Horeh M, Abolhassani F, Jafarpour F, Moini A, Karbalaie K, Hosseini SM, et al. Vitrification at Day3 stage appears not to affect the methylation status of H19/IGF2 differentially methylated region of in vitro produced human blastocysts. Cryobiology. 2016;73:168–74.
Zhao XM, Du WH, Hao HS, Wang D, Qin T, Liu Y, et al. Effect of vitrification on promoter methylation and the expression of pluripotency and differentiation genes in mouse blastocysts. Mol Reprod Dev. 2012;79:445–50.
Capalbo A, Poli M, Jalas C, Forman EJ, Treff NR. On the reproductive capabilities of aneuploid human preimplantation embryos. Am J Hum Genet. 2022;109:P1572-1581.
Groff AF, Resetkova N, DiDomenico F, Sakkas D, Penzias A, Rinn JL, et al. RNA-seq as a tool for evaluating human embryo competence. Genome Res. 2019;29:1705–18.
Cagnone G, Sirard MA. The embryonic stress response to in vitro culture: Insight from genomic analysis. Reproduction. 2016;152:R247–61.
Garcia-Dominguez X, Vicente JS, Marco-Jiménez F. Developmental plasticity in response to embryo cryopreservation: The importance of the vitrification device in rabbits. Animals. 2020;10:804.
Acknowledgements
The authors are grateful to all the embryologists of the IVF lab of IVIRMA Valencia.
Funding
This project was supported by the intramural funds of IVIRMA Global (Valencia), a grant from the IVI foundation (Instituto de Investigación Sanitaria La Fe, Valencia), and a grant for the recruitment of support staff in a technology transfer project (APOTI, Generalitat Valenciana).
Author information
Authors and Affiliations
Contributions
All authors have materially participated in the research and/or article preparation. (1) M. Pérez-Sánchez, ML. Pardiñas, A. Díez-Juan, A. Quiñonero, A. Martin, MJ. de los Santos: the conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) M. Pérez-Sánchez, ML. Pardiñas, F. Dominguez, A. Martin, MJ. de los Santos: drafting the article or revising it critically for important intellectual content. (3) C. Vidal, D. Beltran, A. Mifsud, A. Mercader, A. Pellicer, A. Cobo, MJ. de los Santos: final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of IVI Valencia. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Sánchez, M., Pardiñas, M.L., Díez-Juan, A. et al. The effect of vitrification on blastocyst mitochondrial DNA dynamics and gene expression profiles. J Assist Reprod Genet 40, 2577–2589 (2023). https://doi.org/10.1007/s10815-023-02952-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02952-3